Speak directly to the analyst to clarify any post sales queries you may have.
Healthcare Fixed Pressure Valves Are Transforming Patient Care by Delivering Unwavering Flow Control and Ensuring Safety in Critical Medical Procedures
Healthcare fixed pressure valves are emerging as critical components in modern medical devices, where precision and reliability underpin patient safety and treatment efficacy. These valves regulate gas or fluid flow at predetermined pressures, ensuring consistent performance in settings ranging from anesthesiology to dialysis. Understanding these devices' role in clinical workflows is essential for device manufacturers, hospital procurement teams, and regulatory bodies alike.Over the past decade, advances in materials science, miniaturization, and manufacturing techniques have elevated fixed pressure valves from ancillary parts to strategic assets. They now help reduce system complexity, lower maintenance requirements, and enhance operational safety. As the healthcare industry places increasing emphasis on patient-centric care, the adoption of these valves continues to rise, reflecting a broader commitment to optimizing procedural outcomes through reliable instrumentation.
Rapid Technological Advances and Regulatory Reforms Are Reshaping the Healthcare Fixed Pressure Valve Landscape to Meet Evolving Clinical Demands Worldwide
The healthcare fixed pressure valve landscape has undergone transformative shifts driven by rapid technological innovation and evolving regulatory mandates. Additive manufacturing techniques have enabled complex geometries that enhance valve responsiveness, while advanced polymers and biocompatible coatings improve durability and sterilization compatibility. Concurrently, regulatory bodies have tightened quality and traceability requirements, compelling manufacturers to adopt end-to-end digital documentation and batch tracking solutions.Moreover, emerging digital health initiatives are integrating smart sensors with fixed pressure valves to deliver real-time performance monitoring and predictive maintenance alerts. This convergence of hardware and software is redefining competitive advantage, as device OEMs strive to offer differentiated value propositions. As clinical environments become more data-driven, the ability to deliver seamless interoperability between valves and broader health IT systems will continue to shape market dynamics.
Escalating US Tariffs in 2025 Are Imposing New Cost Structures and Strategic Realignments on Manufacturers of Healthcare Fixed Pressure Valves
The introduction of new US tariffs in 2025 has introduced a pivotal inflection point for fixed pressure valve manufacturers and end users. Import duties on raw materials and finished components have escalated production costs, prompting companies to reassess global supply chains and localize key manufacturing processes. These adjustments are fostering regionalization strategies as firms seek to mitigate the impact of cost volatility and maintain competitive pricing structures.At the same time, end users are beginning to recalibrate procurement strategies. Longer-term contracts and flexible sourcing agreements are gaining traction, allowing healthcare providers to lock in pricing amid geopolitical uncertainty. Meanwhile, some forward-looking manufacturers are exploring alternative materials and design optimizations to offset tariff-driven cost increases, preserving margins without compromising the high reliability standards critical to patient safety.
Segmentation Analysis Uncovers Adoption Trends of Fixed Pressure Valves by Material Type, Pressure Rating, Clinical Application, End User, and Distribution Channel
Segmentation analysis of the fixed pressure valve market reveals pronounced trends in material selection, performance parameters, clinical use cases, user demographics, and sales channels. Based on material type, metallic variants demonstrate superior mechanical strength and thermal tolerance, making them ideal for high-stress applications, while non-metallic options offer cost advantages and enhanced corrosion resistance. Based on pressure rating, valves rated for high pressures are increasingly employed in respiratory care devices, whereas low and medium pressure ratings dominate anesthesia delivery and dialysis systems.Clinical applications further differentiate valve adoption patterns, as anesthesia delivery systems demand ultra-precise flow control, dialysis machines require robust pressure stability under continuous operation, and respiratory devices emphasize rapid response characteristics. Surgical instruments, including electrosurgical units and laparoscopic devices, place unique demands on valve miniaturization and sterility compatibility. Based on end user, hospitals and clinics represent the largest volume segment due to their diverse procedural requirements, whereas ambulatory surgical centers and diagnostic laboratories prioritize compact, low-maintenance solutions. Based on distribution channel, the offline channel remains foundational through established medical device distributors, while online direct sales and distributor networks are gaining momentum for expedited procurement and customized service offerings.
Regional Dynamics in Fixed Pressure Valve Adoption Highlight Contrasting Growth Drivers across the Americas, Europe Middle East Africa, and the Asia-Pacific Markets
Regional dynamics in the fixed pressure valve market reflect the unique healthcare infrastructures and regulatory frameworks across the Americas, Europe Middle East Africa, and Asia-Pacific regions. In the Americas, large integrated health systems and a high share of elective procedures underpin robust demand for advanced valve solutions, particularly within leading hospital networks in the United States. Meanwhile, Latin American markets are gradually expanding through public-private partnerships that prioritize cost-effective precision components.In EMEA, diverse national regulations and reimbursement landscapes drive localized product adaptations. European manufacturers often lead in sustainability and circular economy initiatives, focusing on recyclable materials and extended life-cycle performance. The Middle East and Africa present niche growth pockets in private healthcare expansions, with an emphasis on reliable devices that require minimal infrastructure support. Across Asia-Pacific, dynamic medical tourism and rising chronic disease prevalence fuel investments in both public and private hospitals. Rapid urbanization in emerging economies is accelerating the adoption of fixed pressure valves, particularly in high-throughput facilities.
Competitive Landscape Analysis of Leading Manufacturers Reveals Strategic Partnerships, Innovation Focus, and Positioning in the Fixed Pressure Valve Market
Leading companies in the fixed pressure valve arena are redefining competitive dynamics through strategic partnerships, targeted R&D investments, and portfolio expansions. Several global device OEMs have forged collaborations with specialty materials suppliers to co-develop next-generation valve architectures that balance weight, strength, and biocompatibility. This trend underscores the importance of cross-industry alliances in overcoming material science challenges.Other market leaders are prioritizing digital integration, embedding pressure sensors and connectivity modules to enable predictive maintenance and improve traceability. Additionally, a subset of companies is expanding through selective acquisitions of niche valve specialists, enhancing their ability to offer turnkey flow control solutions. Across the board, innovation pipelines are focusing on miniaturization for minimally invasive procedures and on enhancing sterilization compatibility to meet rigorous hospital standards.
Strategic Imperatives for Industry Leaders to Capitalize on Emerging Opportunities and Navigate Challenges in the Fixed Pressure Valve Landscape
Industry leaders should prioritize end-to-end value chain optimization to maintain cost leadership and quality assurance. By investing in advanced manufacturing platforms and digital quality control systems, organizations can drive efficiency gains while ensuring regulatory compliance. Strategic reshoring initiatives may also help mitigate tariff impacts and reduce supply chain complexity, ultimately improving responsiveness to clinical demand fluctuations.Moreover, forging deeper collaborations with healthcare providers will enable co-creation of tailored valve solutions that address specific procedural needs. Embedding data analytics capabilities into valve platforms can unlock new service models, such as predictive maintenance contracts and usage-based pricing. Finally, companies must remain vigilant on emerging regulatory requirements for sustainability and traceability, leveraging transparent materials sourcing and end-of-life recycling programs to create competitive differentiation.
Robust Research Methodology Underpins the Analysis with Comprehensive Data Collection, Rigorous Validation, and Expert Interviews to Ensure Credibility
The research methodology underpinning this analysis combines rigorous primary and secondary data collection with expert validation to ensure comprehensive coverage and credibility. Initial secondary research encompassed a review of industry publications, regulatory filings, patent databases, and clinical guidelines to establish a broad knowledge base. Primary research then engaged device OEM executives, procurement managers, and clinical engineers through in-depth interviews and structured questionnaires to surface qualitative insights and on-the-ground perspectives.Quantitative data was triangulated through proprietary databases and trade statistics, enabling cross-verification of volume trends and technology adoption patterns. Analytical frameworks, including Porter's Five Forces and SWOT analyses, were applied to assess competitive pressures and strategic positioning. Finally, all findings were vetted through peer review with subject matter experts to validate assumptions, refine projections, and ensure alignment with the latest industry developments.
Conclusive Insights Summarize the Transformation, Challenges, and Growth Imperatives of Healthcare Fixed Pressure Valves in a Rapidly Evolving Industry
In summary, healthcare fixed pressure valves have evolved from simple flow regulators to critical enablers of patient safety and device performance. The convergence of material innovations, digital integration, and regional supply chain realignments underscores the market's dynamic nature. Navigating the impact of new tariff regimes, regulatory shifts, and emerging clinical demands will require agility and strategic foresight.Market leaders who invest in advanced manufacturing, collaborative R&D, and data-driven service models are poised to capture disproportionate gains. As the industry continues to prioritize precision medicine and minimally invasive interventions, the role of fixed pressure valves will only grow in importance. Stakeholders who harness these insights will be well positioned to drive the next wave of innovation and deliver enhanced value to healthcare providers and patients alike.
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Material Type
- Metalic
- Non Metalic
- Pressure Rating
- High Pressure
- Low Pressure
- Medium Pressure
- Application
- Anesthesia Delivery Systems
- Dialysis Machines
- Respiratory Care Devices
- Surgical Instruments
- Electrosurgical units
- Laparoscopic devices
- End User
- Ambulatory Surgical Centers
- Diagnostic Laboratories
- Hospitals & Clinics
- Research & Academic Institutions
- Distribution Channel
- Offline
- Online
- Direct Sale
- Distributor Network
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- B. Braun Melsungen AG
- Natus Medical Incorporated
- Adamant Valves by Oceania International LLC
- AVC India Pvt Ltd
- Bicakcilar
- Delta Surgical Ltd.
- Desu Medical
- Integra LifeSciences Corporation
- Medtronic plc
- Möller Medical GmbH
- Promedon GmbH
- Sophysa
- Sophysa S.A.S.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Healthcare Fixed Pressure Valves market report include:- B. Braun Melsungen AG
- Natus Medical Incorporated
- Adamant Valves by Oceania International LLC
- AVC India Pvt Ltd
- Bicakcilar
- Delta Surgical Ltd.
- Desu Medical
- Integra LifeSciences Corporation
- Medtronic PLC
- Möller Medical GmbH
- Promedon GmbH
- Sophysa
- Sophysa S.A.S.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 195 |
| Published | November 2025 |
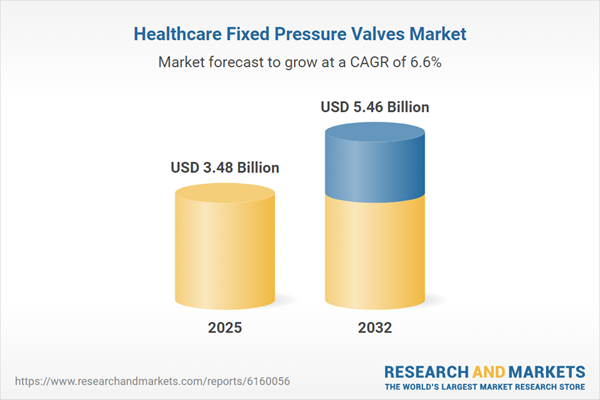
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 3.48 Billion |
| Forecasted Market Value ( USD | $ 5.46 Billion |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 14 |