Speak directly to the analyst to clarify any post sales queries you may have.
Unearthing the Vital Role of Bulk Paclitaxel in Oncology and Pharmaceutical Manufacturing to Drive Precision and Therapeutic Breakthroughs
Bulk paclitaxel has emerged as one of the most critical active pharmaceutical ingredients in contemporary oncology, underpinning numerous chemotherapy protocols for malignancies such as breast, lung, and ovarian cancers. As a microtubule-stabilizing agent derived from the bark of the Pacific yew tree, paclitaxel's molecular mechanism disrupts mitosis, thereby inhibiting tumor cell proliferation. This compound's significance extends beyond its clinical efficacy; it represents a focal point for innovation across extraction, synthesis, and formulation science.In recent years, supply chain resilience and manufacturing scalability have become paramount concerns for stakeholders in both pharmaceutical companies and research institutes. Divergent approaches incorporating plant extraction and semi-synthetic pathways have shaped availability, cost structures, and sustainability metrics. With semi-synthetic processes mitigating some constraints of natural sourcing, industry players have pursued hybrid strategies to ensure consistent supply while addressing environmental considerations.
Meanwhile, advances in formulation technologies continue to unlock new therapeutic avenues. Traditional injection solutions have evolved to include nanoparticle-based delivery systems and dry powder formats, each designed to enhance bioavailability, reduce infusion-related toxicity, and broaden clinical applications. As research investments intensify, the role of bulk paclitaxel has expanded from a single therapeutic focus to a versatile platform supporting next-generation targeted therapies.
Navigating Transformative Technological Shifts and Innovative Formulation Advances That Are Shaping the Future of Bulk Paclitaxel Production
The landscape of bulk paclitaxel production has been reshaped by transformative shifts in both technology and regulatory frameworks. Rapid innovation in extraction methodologies, including supercritical fluid techniques and enzyme-assisted isolation, has elevated yield efficiency and reduced ecological footprint. Concurrently, breakthroughs in semi-synthetic routes-leveraging precursor compounds derived from renewable feedstocks-have improved supply chain robustness and minimized dependency on limited natural sources.Formulation advancements have played an equally pivotal role. While conventional injection formulations remain widely used, nanoparticle-enabled systems are gaining traction for their capacity to improve targeted delivery and mitigate adverse effects. Dry powder formats have also emerged as viable alternatives, facilitating more flexible storage and administration protocols. This expansion of formulation modalities has been propelled by concerted research efforts, incentivized by regulatory bodies that encourage innovation to meet evolving clinical needs.
Regulatory shifts have further catalyzed this evolution. Accelerated approval pathways for oncology therapeutics have prompted manufacturers to invest in advanced characterization and quality control techniques. Moreover, greater emphasis on sustainability and traceability has led to the adoption of blockchain-based systems for raw material certification, ensuring compliance across global supply chains. Together, these transformative dynamics underscore a future in which bulk paclitaxel is not only a critical therapeutic agent but also a case study in agile, technology-driven pharmaceutical manufacturing.
Assessing the Cumulative Economic Impact of Newly Imposed United States Tariffs on Bulk Paclitaxel Supply Chains and Trade Dynamics
The imposition of new United States tariffs on bulk paclitaxel imports in 2025 has introduced a complex set of challenges for global supply chains. Tariff rates applied across key precursor materials and finished bulk shipments have had ripple effects on cost structures, compelling stakeholders to evaluate sourcing strategies more rigorously. Wholesalers and contract manufacturers are adjusting procurement timelines, seeking to optimize cushion stock while negotiating revised pricing models with suppliers.These financial pressures have driven an uptick in localized production initiatives, with certain manufacturers accelerating investments in semi-synthetic pathways to reduce reliance on imports. At the same time, research institutes and pharmaceutical companies are reassessing their inventory management protocols, balancing the need for uninterrupted clinical supply against the increased carrying costs of higher stock levels. Buyers have also explored collaborative purchasing agreements to leverage volume commitments, spreading tariff impacts across consortium partners.
Transitional adaptations in regulatory compliance have further complicated the environment. Importers must navigate revised classification criteria and documentation requirements, which in turn influence lead times and logistical planning. In response, some stakeholders have implemented advanced trade analytics platforms to anticipate tariff triggers and streamline customs processes. Collectively, these measures illustrate the cumulative impact of tariff policies on the intricate ecosystem supporting bulk paclitaxel accessibility and affordability.
Extracting Key Insights from Comprehensive Segmentation Analysis Revealing Source, Formulation Type, Purity Grade, Application, End User, and Sales Channel Dynamics
An in-depth segmentation analysis reveals the nuanced dynamics governing the bulk paclitaxel ecosystem, beginning with the dichotomy between plant extraction and semi-synthetic sources. While plant extraction remains foundational for certain legacy supply pathways, semi-synthetic techniques have gained prominence due to their scalability and alignment with sustainability mandates. This divergence in source methodologies directly influences downstream formulation choices and quality assurance frameworks.The spectrum of formulation types further underscores this complexity. Injection formulations continue to dominate established clinical settings, yet the emergence of nanoparticle platforms is redefining delivery paradigms by enabling targeted biodistribution and reduced systemic toxicity. Dry powder variants enhance stability and extend shelf life, offering logistical advantages for distribution in resource-constrained geographies. As these modalities proliferate, the interplay between formulation choice and therapeutic application becomes increasingly strategic.
Purity grades represent another critical axis of differentiation. Analytical grade paclitaxel caters to rigorous research and diagnostic applications, while pharmaceutical grade material adheres to stringent pharmacopoeial standards for human administration. This duality shapes production workflows, quality control protocols, and regulatory submissions. Meanwhile, the breadth of clinical applications-from breast cancer and Kaposi's sarcoma to lung and ovarian malignancies-drives tailored formulation research and influences prioritization of end users, including hospitals, pharmaceutical companies, and research institutes.
Finally, the sales channel dimension encapsulates the evolving interface between producers and consumers. Traditional offline distribution channels remain integral for large-scale institutional procurement, whereas online platforms are gaining traction for expedited order fulfillment and enhanced transparency. This bifurcation requires companies to develop integrated channel strategies that balance compliance considerations with customer convenience and service level efficiency.
Illuminating Regional Footprints in the Bulk Paclitaxel Landscape Spanning the Americas, Europe, Middle East & Africa, and Asia-Pacific Spheres
Regional dynamics in the bulk paclitaxel domain are shaped by distinct regulatory regimes, manufacturing capabilities, and therapeutic demand profiles across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, a robust biotechnology infrastructure and favorable intellectual property protection have fostered advanced research collaborations. Leading contract manufacturing organizations in this region have leveraged capacity expansions to meet both domestic and export requirements, positioning the Americas as a pivotal distribution hub.In Europe, Middle East & Africa, stringent regulatory frameworks and harmonized pharmacopoeial standards have driven manufacturers to implement high-precision quality control measures. This region places a premium on traceability and compliance, with certifying agencies enforcing rigorous batch release protocols. Meanwhile, emerging markets within the Middle East and Africa have begun to invest in local production capabilities, signaling a long-term shift toward greater regional self-sufficiency.
The Asia-Pacific region has experienced pronounced growth in bulk paclitaxel production, propelled by competitive manufacturing costs, government incentives, and expanding pharmaceutical R&D. Several countries have scaled up semi-synthetic extraction facilities and embraced innovative formulation research. As clinical trial activity intensifies across the region, the Asia-Pacific landscape is poised to become a primary source of both high-volume supply and pioneering therapeutic development.
Profiling Leading Industry Stakeholders and Their Strategic Innovations Driving Advancements in Bulk Paclitaxel Research, Development, and Commercialization
Leading stakeholders in the bulk paclitaxel arena are characterized by strategic investments in capacity augmentation, advanced R&D pipelines, and innovative collaboration models. Several prominent API manufacturers have forged alliances with biotech firms to co-develop nanoparticle delivery platforms, sharing risk and intellectual property to accelerate time-to-clinic. At the same time, key contract development organizations have upgraded manufacturing suites to accommodate both analytical and pharmaceutical grade production, ensuring compliance with evolving global standards.Research institutes are partnering with commercial entities to validate novel extraction and purification techniques, while pharmaceutical companies are extending their product portfolios through tuck-in acquisitions and licensing agreements. End-to-end supply chain integration-from precursor sourcing to final product distribution-has become a hallmark of the most successful players, who employ digital traceability solutions and real-time analytics to optimize operations. This emphasis on end-user responsiveness and regulatory alignment underscores the competitive differentiation strategies shaping the bulk paclitaxel sector.
Actionable Strategic Recommendations for Industry Leaders to Optimize Supply Chains, Enhance Production Efficiencies, and Foster Collaborative Innovation in Bulk Paclitaxel
To navigate the evolving bulk paclitaxel ecosystem, industry leaders should prioritize diversification of raw material sources, balancing plant extraction efforts with semi-synthetic investments to bolster supply resilience. Concurrently, expanding nanoparticle formulation capabilities will unlock new therapeutic applications and differentiate product offerings. Strengthening online sales channels alongside traditional distribution networks can enhance responsiveness to urgent clinical demand while fostering transparency and customer engagement.Investment in trade analytics and customs compliance tools will mitigate the financial impacts of tariff fluctuations, enabling more precise cost management. Leaders should also seek collaborative research partnerships with academic institutions and contract laboratories to accelerate process innovation and share development risks. Finally, proactive engagement with regulatory bodies-through fast-track designation requests and participation in standards committees-will position organizations to capitalize on emerging approval pathways and shape future policy directions.
Unveiling Rigorous Multi-Phase Research Methodology Incorporating Primary Interviews, Secondary Data Triangulation, and Robust Quality Assurance Protocols
This research study employed a multi-phase methodology, beginning with comprehensive secondary data analysis of scientific literature, regulatory filings, and industry publications to establish a foundational understanding of bulk paclitaxel production and application trends. Building upon these insights, primary interviews were conducted with senior executives from manufacturing organizations, oncology research leaders, and procurement specialists to capture firsthand perspectives on supply chain dynamics and innovation priorities.Data triangulation techniques were applied to reconcile discrepancies between secondary sources and interview findings, ensuring that conclusions reflect both quantitative metrics and qualitative context. Rigorous quality assurance protocols-including peer review by subject matter experts and cross-validation against benchmark studies-were integrated throughout the research process. Finally, stakeholder workshops were convened to refine strategic frameworks and validate actionable recommendations, enhancing the study's relevance and practical utility for decision-makers.
Synthesis of Core Findings Highlighting Critical Trends, Challenges, and Opportunities Within the Bulk Paclitaxel Arena for Informed Decision-Making
The analysis highlights the critical interplay of source innovation, formulation diversification, and regulatory adaptation in shaping the bulk paclitaxel landscape. Technological advances in both extraction and semi-synthetic synthesis have enhanced supply chain stability, while novel delivery platforms are redefining clinical applications. The imposition of tariffs has underscored the importance of strategic sourcing and trade analytics, prompting shifts toward localized production and consortium purchasing models.Segmented insights reveal that differences in purity grade requirements, end-user profiles, and sales channels necessitate tailored operational strategies. Regionally, the Americas, Europe, Middle East & Africa, and Asia-Pacific each present distinct opportunities and challenges driven by infrastructure maturity, regulatory stringency, and cost competitiveness. Leading companies are responding with integrated supply chain solutions, targeted R&D alliances, and digital traceability innovations.
In conclusion, success in this dynamic environment will hinge on organizations' abilities to synchronize technological agility, regulatory foresight, and strategic collaboration. By leveraging the comprehensive insights presented here, stakeholders can make informed decisions to navigate complexity and capitalize on emerging growth avenues within the global bulk paclitaxel domain.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Source
- Plant Extraction
- Semi Synthetic
- Formulation Type
- Injection
- Nanoparticle Formulation
- Powder
- Purity Grade
- Analytical Grade
- Pharmaceutical Grade
- Application
- Breast Cancer
- Kaposi's Sarcoma
- Lung Cancer
- Ovarian Cancer
- End User
- Hospitals
- Pharmaceutical Companies
- Research Institutes
- Sales Channel
- Offline
- Online
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Alchem International
- Apino Pharma Co., Ltd.
- Avanscure Lifesciences Pvt. Ltd.
- AXXO GmbH
- Cipla Limited
- Fresenius Kabi AG
- Guilin Huiang Biochemistry Pharmaceutical Co., Ltd.
- Hefei Home Sunshine Pharmaceutical Technology Co., Ltd.
- Indena S.p.A.
- Intas Pharmaceuticals Ltd.
- Intelicure Lifesciences
- LGM Pharma
- Panacea Biotec Limited
- PHYTON LTD
- Rochem International, Inc.
- Samyang Holdings Corporation
- Sinoway industrial Co.,Ltd
- Teva API, Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Bulk Paclitaxel market report include:- Alchem International
- Apino Pharma Co., Ltd.
- Avanscure Lifesciences Pvt. Ltd.
- AXXO GmbH
- Cipla Limited
- Fresenius Kabi AG
- Guilin Huiang Biochemistry Pharmaceutical Co., Ltd.
- Hefei Home Sunshine Pharmaceutical Technology Co., Ltd.
- Indena S.p.A.
- Intas Pharmaceuticals Ltd.
- Intelicure Lifesciences
- LGM Pharma
- Panacea Biotec Limited
- PHYTON LTD
- Rochem International, Inc.
- Samyang Holdings Corporation
- Sinoway industrial Co.,Ltd
- Teva API, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
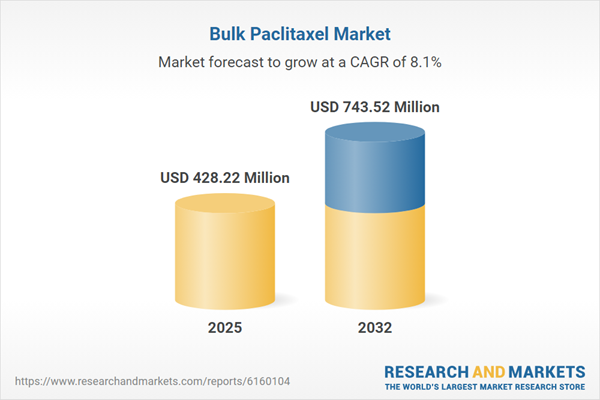
| Estimated Market Value ( USD | $ 428.22 Million |
| Forecasted Market Value ( USD | $ 743.52 Million |
| Compound Annual Growth Rate | 8.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 19 |