1h Free Analyst Time
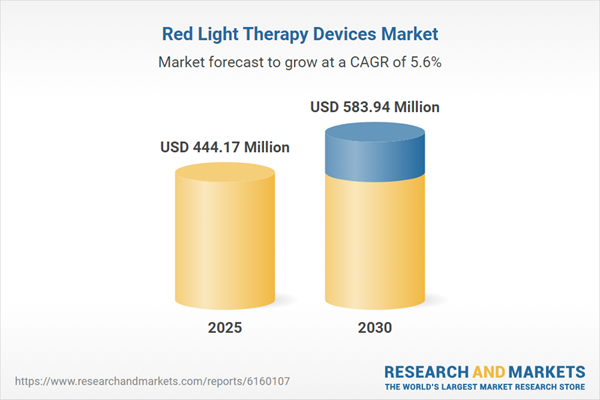
The Red Light Therapy Devices Market grew from USD 421.38 million in 2024 to USD 444.17 million in 2025. It is expected to continue growing at a CAGR of 5.58%, reaching USD 583.94 million by 2030. Speak directly to the analyst to clarify any post sales queries you may have.
Illuminating the Rise of Red Light Therapy Solutions
Red light therapy devices have rapidly transitioned from niche wellness solutions to mainstream therapeutic tools embraced by consumers, clinicians, and sports professionals alike. This modality leverages specific wavelengths of visible red light to penetrate skin layers and stimulate cellular activity, harnessing benefits that range from accelerated tissue repair to enhanced collagen production. The growing emphasis on non-invasive, drug-free therapies has energized demand, driving manufacturers to innovate across form factors and power outputs.As more scientific evidence underpins efficacy claims, awareness among key stakeholders has surged. Clinics and hospitals are integrating red light beds and panels into rehabilitation protocols, while at-home offerings such as masks and wands have democratized access for everyday users. Amid this dynamic, the influx of new entrants has fueled competition, prompting established players to refine their product portfolios and reinforce safety and performance standards. In turn, regulatory bodies have started to take notice, exploring guidelines to ensure consistent quality and consumer protection.
Consequently, an inflection point is underway where technological maturity intersects with heightened consumer expectations. This introduction sets the stage for an in-depth examination of transformative shifts, tariff implications, segmentation insights, regional nuances, leading competitors, strategic recommendations, and a transparent research methodology that collectively illuminate the red light therapy device market’s current state and near-term trajectory.
Evolving Technologies and Consumer Expectations Reshaping Therapy Landscape
An array of transformative shifts is redefining the red light therapy landscape, from technological advancements to evolving consumer preferences. The maturation of LED-based platforms alongside traditional laser systems has broadened the spectrum of therapeutic applications. Where laser technology once dominated clinical settings with its precise wavelength control, high-power LED panels and handheld wands are gaining favor for their cost-effectiveness and versatility. As a result, product roadmaps now emphasize hybrid designs that merge LED arrays with targeted laser diodes, delivering both coverage and focused energy to treat diverse tissue depths.Simultaneously, the convergence of digital health is catalyzing new experiences. Wireless connectivity, mobile applications, and integrated sensors allow users to track session parameters, monitor skin responses, and receive personalized treatment recommendations. This shift towards data-driven therapy not only elevates user engagement but also fosters long-term adherence through feedback loops and telehealth integration. In parallel, demand for at-home solutions has skyrocketed as consumers seek convenience without sacrificing clinical-grade performance.
Regulatory landscapes are evolving in lockstep with technological innovation. Agencies worldwide are exploring frameworks that differentiate medical devices from wellness products, seeking to balance consumer access with safety oversight. This evolving definition of red light therapy devices is prompting manufacturers to proactively engage with regulators and invest in clinical validation studies. Ultimately, these shifts are forging a market where device capabilities, digital connectivity, and regulatory compliance coalesce to deliver compelling value propositions.
Assessing the Ripple Effect of New US Tariffs on Device Supply Chains
The introduction of new tariffs in 2025 has reverberated across global supply chains, imposing additional costs on imported components and finished devices. Manufacturers that rely on overseas production faced immediate pressure to reassess logistics strategies and absorb margin contractions. Many opted to redistribute procurement across alternative regions or renegotiate supplier contracts to mitigate cost hikes. At the same time, device pricing adjustments began to emerge in key markets, testing consumer elasticity and compelling some vendors to reposition offerings within premium and value tiers.Moreover, the tariff landscape has accelerated conversations around near-shoring and vertical integration. Several leading companies are exploring local assembly initiatives to bypass import levies and strengthen control over quality assurance. While upfront capital investment has increased, the potential for reduced lead times and enhanced responsiveness to market fluctuations offers a strategic advantage. Over the long term, this paradigm shift may foster more resilient manufacturing networks and create opportunities for regional partnerships, particularly in North America.
Crucially, distributors and end users have also adapted their procurement strategies. Bulk orders were staggered ahead of tariff implementations, resulting in short-term inventory buildups and subsequent demand normalization. Going forward, dynamic pricing models and contract clauses that share tariff risks between manufacturers, distributors, and buyers are becoming more commonplace. These evolving arrangements reflect a broader trend of collaborative risk management that will shape the red light therapy device ecosystem well beyond 2025.
Unpacking Market Diversity Through Key Segmentation Lenses
A nuanced understanding of market segmentation reveals the multifaceted nature of red light therapy demand. Examining the market through the prism of product types uncovers distinct user preferences for full-body beds that serve clinical and high-end spa environments, compact masks tailored to facial treatments, versatile panels suited for home and clinical settings, and portable wands favored by sports and fitness enthusiasts seeking targeted relief. Each form factor commands unique design and regulatory considerations, influencing manufacturing complexity and go-to-market strategies.Shifting focus to underlying technology delineates two core streams: laser solutions prized for their precise wavelength outputs and deep tissue penetration, and LED platforms celebrated for their broader coverage, energy efficiency, and cost-effectiveness. The interplay between these technologies has given rise to hybrid devices that aim to capture the benefits of both, driving competitive innovation while also raising calibration and safety imperatives.
Application-based segmentation further illustrates the spectrum of therapeutic endpoints. Hair growth regimens leverage specific wavelengths to stimulate follicles, while muscle recovery devices integrate with athletic recovery protocols. Pain management applications span joint pain relief in clinical settings to muscle pain alleviation for individual users, each requiring tailored dosage parameters. Within the skin treatment domain, acne therapy protocols, anti-aging regimens, and specialized wound healing devices highlight the modality’s versatility. These distinct use cases necessitate targeted clinical validation, influencing regulatory classification and insurance reimbursement potential.
Understanding end-user segmentation provides insight into distribution and marketing channels. Clinic operators evaluate devices on throughput and return on investment, home users prioritize ease of use and safety features, hospitals demand rigorous medical certifications, and spa wellness centers seek products that elevate customer experiences. Distribution channels mirror this diversity, with offline retail encompassing pharmacies and specialty stores, and online sales flowing through brand websites and third-party e-commerce platforms. Each channel imposes its own set of compliance, logistics, and promotional requirements, underscoring the importance of channel-specific strategies for market penetration and sustained growth.
Regional Dynamics Driving Global Red Light Therapy Adoption
Regional dynamics exert profound influence over the red light therapy device market, shaping both demand drivers and competitive landscapes. In the Americas, strong consumer spending power and entrenched wellness cultures have fueled adoption across clinical and at-home channels. The United States serves as a bellwether, where reimbursement policies and clinical guideline endorsements elevate the modality’s credibility and expedite uptake in physical therapy clinics and sports medicine centers. Meanwhile, emerging markets in Latin America present promising growth corridors as awareness campaigns and distributor partnerships expand access.Turning to Europe, Middle East & Africa, regulatory harmonization efforts in the European Union are streamlining device approvals, enabling manufacturers to leverage single-market authorizations. In contrast, the Middle East is witnessing a premiumization trend within luxury spas and medical tourism hubs, where high-end beds and panels command significant investment. Sub-Saharan Africa remains nascent but shows potential for point-of-care adoption in urban medical facilities, provided affordability and infrastructure barriers can be addressed.
In Asia-Pacific, rapid urbanization, rising disposable incomes, and an affinity for cutting-edge health technologies are accelerating adoption. Japan and South Korea lead with well-established medical device industries and supportive regulatory frameworks that encourage innovation. China’s massive consumer base and local manufacturing capabilities are driving competitive pricing and volume-driven growth, while India’s burgeoning wellness sector and expanding telehealth platforms create new channels for device distribution. Each region’s distinct combination of economic conditions, healthcare systems, and cultural attitudes toward wellness underscores the importance of tailored market entry and expansion strategies.
Competitive Landscapes and Leading Innovators in Red Light Therapy Space
The competitive landscape is characterized by a blend of established medical device manufacturers, specialized wellness brands, and nimble startups. Leading incumbents differentiate through comprehensive portfolios that span bed, panel, mask, and wand offerings, allowing them to address multiple end-user segments and cross-sell within existing networks. These firms often emphasize robust clinical validation, investing in controlled trials to substantiate efficacy claims and secure regulatory approvals in major markets.Meanwhile, emerging players are redefining value propositions by integrating smart connectivity and user-centric design elements. They are forging partnerships with dermatology clinics and physical therapy centers to pilot novel device formats, leveraging real-world evidence to refine treatment protocols. Some innovators focus on proprietary waveform modulation or combined light-and-vibration technologies to enhance therapeutic outcomes, captivating discerning practitioners and tech-savvy consumers.
Beyond product differentiation, competitive success hinges on supply chain agility and channel partnerships. Companies that have diversified manufacturing footprints and cultivated relationships with leading e-commerce platforms have managed to navigate tariff disruptions and shifting consumer behaviors. Strategic alliances with telecom providers for mobile app integration or with academic institutions for joint research further distinguish certain vendors. Overall, a mix of scale, specialization, and ecosystem collaboration defines the leaders vying for market share in this rapidly evolving domain.
Strategic Imperatives for Market Leadership and Sustainable Growth
Industry leaders seeking to capitalize on red light therapy’s momentum must pursue a series of strategic imperatives. First, allocating resources toward advanced R&D will be critical for refining device efficacy and safety. Investment should focus on optimizing wavelength outputs for specific applications and exploring hybrid energy modalities that combine light therapy with complementary physical or acoustic interventions. By anchoring innovation in clinical evidence, companies can bolster their value propositions and differentiate in a crowded marketplace.Second, strengthening supply chain resilience is paramount. Diversifying manufacturing locations and forging local assembly partnerships can mitigate tariff-related cost pressures and reduce lead times. Implementing dynamic pricing models and contractual mechanisms that share risk across the value chain will help maintain margin stability while preserving competitive price points.
Third, forging strategic alliances with healthcare providers, research institutions, and digital health platforms will amplify market reach. Co-development initiatives and pilot programs in clinical settings not only generate compelling case studies but also accelerate regulatory approvals and insurance reimbursement pathways. Concurrently, integrating device control and performance tracking into mobile applications will enhance user engagement and enable long-term outcome tracking, thereby driving repeat sales and subscription-based service models.
Lastly, a nuanced channel strategy is essential. Premium clinical-grade devices should align with medical distributors and hospital procurement processes, while consumer-focused products must leverage omnichannel retail approaches that blend brand-owned e-commerce with select specialty stores. Tailoring messaging to emphasize evidence-based benefits, safety features, and ease of use will resonate across diverse end-user segments and foster both trial and loyalty.
Robust Methodological Framework Underpinning Comprehensive Insights
This comprehensive examination is grounded in a robust methodological framework designed to deliver objective and actionable insights. The research process commenced with extensive secondary analysis of publicly available data, including regulatory filings, patent databases, and industry publications. This foundation was enriched through primary interviews with key stakeholders, encompassing device manufacturers, clinical practitioners, distributors, and end users, to capture diverse perspectives on market dynamics.Quantitative data was triangulated through cross-validation of trade statistics, financial disclosures, and import-export records, enabling precise identification of tariff impacts and supply chain adjustments. The segmentation analysis employed both top-down and bottom-up approaches to ensure consistency across product types, technologies, applications, end users, and distribution channels. Regional assessments incorporated macroeconomic indicators, healthcare infrastructure metrics, and localized consumer behavior studies to contextualize growth drivers and barriers.
Competitive benchmarking leveraged proprietary databases to map company portfolios, R&D pipelines, strategic partnerships, and distribution footprints. Rigorous data quality protocols, including consistency checks and peer reviews, were applied throughout the research lifecycle. The culmination of this methodology is a defensible, nuanced portrayal of the red light therapy device market, providing stakeholders with clarity to inform strategic decision-making.
Synthesis of Red Light Therapy Market Trajectories and Outlook
In synthesizing the varied threads of this executive summary, several core themes emerge. Technological innovation is propelling red light therapy devices across new frontiers of usability and efficacy, while tariff-induced supply chain realignments are prompting more resilient manufacturing strategies. A thorough segmentation lens reveals the distinct demands of product form factors, therapeutic applications, and distribution channels, underscoring the need for tailored strategies.Regionally, the Americas, Europe, Middle East & Africa, and Asia-Pacific each offer unique opportunities shaped by regulatory environments, consumer attitudes, and healthcare infrastructures. Competitive positioning is equally nuanced, with incumbent manufacturers and agile newcomers vying for leadership through differentiated portfolios, digital integrations, and strategic partnerships. To thrive in this dynamic landscape, industry participants must marry innovation with operational excellence, investing in clinical validation, supply chain flexibility, and channel optimization.
Ultimately, the red light therapy device market stands at a pivotal juncture. Stakeholders equipped with a granular understanding of transformative shifts, tariff implications, segmentation insights, regional dynamics, and competitive strategies will be best positioned to drive growth and capture emerging opportunities.
Market Segmentation & Coverage
This research report categorizes to forecast the revenues and analyze trends in each of the following sub-segmentations:- Product Type
- Bed
- Mask
- Panel
- Wand
- Technology
- Laser
- LED
- Application
- Hair Growth
- Muscle Recovery
- Pain Management
- Joint Pain
- Muscle Pain
- Skin Treatment
- Acne Treatment
- Anti-Aging
- Wound Healing
- End User
- Clinic
- Home Use
- Hospital
- Spa Wellness Center
- Distribution Channel
- Offline Retail
- Pharmacies
- Specialty Stores
- Online Retail
- Brand Website
- E-Commerce Platform
- Offline Retail
- Americas
- United States
- California
- Texas
- New York
- Florida
- Illinois
- Pennsylvania
- Ohio
- Canada
- Mexico
- Brazil
- Argentina
- United States
- Europe, Middle East & Africa
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- United Arab Emirates
- Saudi Arabia
- South Africa
- Denmark
- Netherlands
- Qatar
- Finland
- Sweden
- Nigeria
- Egypt
- Turkey
- Israel
- Norway
- Poland
- Switzerland
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Philippines
- Malaysia
- Singapore
- Vietnam
- Taiwan
- Lumenis Ltd
- Joovv Inc
- Erchonia Corporation
- Apira Science Inc
- Vielight Inc
- Beurer GmbH
- PlatinumLED Therapy Lights LLC
- Red Light Rising Ltd
- Mito Red Light Inc
- BON CHARGE
- Celluma by BioPhotas LLC
- Dgyao
- EMR-TEK
- Johari Digital Healthcare Ltd.
- Kala Therapy Inc.
- Light Tree Ventures Europe BV
- LightStim International, Inc.
- NORLANYA TECHNOLOGY CO., LIMITED
- NutriJa Lifesciences
- Rollins International Pvt. Ltd.
- Shenzhen Redluxe Technology Co., Ltd.
- ShineNova
- Solawave
- The Wellness Co.
- Wolf Nutrition
- Wuhan Kolda Medical Technology
Table of Contents
1. Preface
2. Research Methodology
3. Executive Summary
4. Market Overview
5. Market Dynamics
6. Market Insights
7. Cumulative Impact of United States Tariffs 2025
8. Red Light Therapy Devices Market, by Product Type
9. Red Light Therapy Devices Market, by Technology
10. Red Light Therapy Devices Market, by Application
11. Red Light Therapy Devices Market, by End User
12. Red Light Therapy Devices Market, by Distribution Channel
13. Americas Red Light Therapy Devices Market
14. Europe, Middle East & Africa Red Light Therapy Devices Market
15. Asia-Pacific Red Light Therapy Devices Market
16. Competitive Landscape
18. ResearchStatistics
19. ResearchContacts
20. ResearchArticles
21. Appendix
List of Figures
List of Tables
Samples

LOADING...
Companies Mentioned
The companies profiled in this Red Light Therapy Devices market report include:- Lumenis Ltd
- Joovv Inc
- Erchonia Corporation
- Apira Science Inc
- Vielight Inc
- Beurer GmbH
- PlatinumLED Therapy Lights LLC
- Red Light Rising Ltd
- Mito Red Light Inc
- BON CHARGE
- Celluma by BioPhotas LLC
- Dgyao
- EMR-TEK
- Johari Digital Healthcare Ltd.
- Kala Therapy Inc.
- Light Tree Ventures Europe BV
- LightStim International, Inc.
- NORLANYA TECHNOLOGY CO., LIMITED
- NutriJa Lifesciences
- Rollins International Pvt. Ltd.
- Shenzhen Redluxe Technology Co., Ltd.
- ShineNova
- Solawave
- The Wellness Co.
- Wolf Nutrition
- Wuhan Kolda Medical Technology
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 196 |
| Published | August 2025 |
| Forecast Period | 2025 - 2030 |
| Estimated Market Value ( USD | $ 444.17 Million |
| Forecasted Market Value ( USD | $ 583.94 Million |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 27 |