Speak directly to the analyst to clarify any post sales queries you may have.
Pioneering Fluorescence Technologies to Revolutionize Medical Devices and Enhance Diagnostic and Therapeutic Capabilities
Fluorescence in medical devices is reshaping the way clinicians detect diseases and guide interventions by harnessing the unique properties of light-emitting molecules. This technology has evolved rapidly from niche laboratory applications to versatile platforms that enhance visualization, improve diagnostic precision, and enable targeted therapies. As the demand for minimally invasive procedures and real-time feedback grows, fluorescence-based solutions are becoming vital components of modern healthcare.Over the past decade, breakthroughs in dye chemistry and imaging sensors have significantly expanded the clinical utility of fluorescence. Novel fluorophores with higher quantum yields and tailored emission profiles have unlocked new possibilities in surgical navigation and in vivo diagnostics. At the same time, advances in miniaturization and signal processing have made portable and integrated systems more practical, opening the door for broader adoption in point-of-care settings.
Looking ahead, the convergence of fluorescence imaging with artificial intelligence and theranostics promises to deliver even greater impact. By analyzing complex fluorescence signatures in real time, AI-driven platforms can expedite decision-making and personalize interventions. Consequently, stakeholders across the medical device value chain are racing to integrate these innovations into next-generation products. This introduction sets the stage for a comprehensive examination of how fluorescence technologies are revolutionizing medical devices and shaping future clinical workflows.
Embracing Next Generation Fluorescence Platforms as a Catalyst for Transformative Shifts in Medical Device Diagnostics and Therapeutics
In recent years, the landscape of fluorescence-enabled medical devices has witnessed a profound transformation driven by technological convergence and evolving clinical demands. Beyond simple marker detection, state-of-the-art platforms now integrate multispectral fluorescence with high-resolution imaging sensors to offer unparalleled tissue differentiation during complex surgical procedures. Moreover, the synergy between fluorescence spectroscopy and machine learning algorithms has unlocked deeper insights into cellular and molecular processes, enabling earlier disease detection and more accurate prognoses.Simultaneously, industry dynamics are shifting as partnerships between device manufacturers, dye developers, and software innovators intensify. Collaboration across these domains is fostering the creation of all-in-one systems that streamline workflows and reduce operational complexity. In parallel, regulatory frameworks are adapting to the growing importance of fluorescence diagnostics, with agencies providing clearer pathways for approval while emphasizing safety and clinical validation.
As a result of these forces, fluorescence applications are expanding beyond traditional specialties such as oncology and ophthalmology into cardiovascular intervention and neurological surgery. These computationally enhanced imaging modalities are redefining intraoperative guidance, paving the way for personalized treatments that optimize patient outcomes. In this section, we explore the key drivers behind these transformative shifts and how they are setting the stage for a new era in medical device innovation.
Unveiling the Comprehensive Impact of United States Tariffs Enacted in 2025 on Fluorescence Enabled Medical Device Innovation and Cost Structures
The implementation of new Tariffs in the United States in 2025 has introduced a significant inflection point for the global supply chain of fluorescence-based medical devices. These measures have increased costs for imported fluorophores, optical filters, and precision sensors, prompting manufacturers to reevaluate sourcing strategies. Consequently, some companies have accelerated nearshoring initiatives, seeking partnerships with domestic chemical producers to ensure stable access to critical raw materials.At the same time, the tariff adjustments have sparked innovation in alternative dye chemistries, with research institutions and specialty chemical firms racing to develop comparable compounds that can be produced locally. This trend is fostering a renewed emphasis on proprietary formulations and reinforcing the importance of patent portfolios. Meanwhile, suppliers of imaging modules and detection hardware have begun to optimize product designs to minimize component costs without compromising performance or safety.
Although these tariff-induced shifts have created short-term pricing pressures, they are also driving resilience across the industry. By diversifying supplier networks and investing in regional manufacturing capabilities, device makers are reducing dependency on a single geographic source. This recalibration not only mitigates risk but also enhances supply chain transparency and sustainability. Moving forward, the cumulative impact of these tariffs will continue to reshape cost structures and competitive dynamics within the fluorescence medical device ecosystem.
Illuminating Market Dynamics through an Integrated Segmentation Lens across Technology Fluorophore Type Application and End User Perspectives
Understanding the market's underlying structure requires a multifaceted segmentation approach that captures variations in technology, fluorophore type, application, and end users. Based on Technology, the market encompasses advanced fluorescence imaging systems equipped with multispectral capabilities, fluorescence spectroscopy platforms designed for biochemical analysis, and fluorescent dyes and agents that serve as the molecular foundation for all applications. Further delineation by fluorophore type reveals distinct preferences for endogenous fluorophores, which leverage naturally occurring molecules for label-free imaging, and exogenous fluorophores, which offer customizable optical properties for targeted diagnostics.Delving deeper into Application, fluorescence in catheters is gaining traction for guiding vascular interventions, while fluorescence-guided surgery spans critical fields such as cardiovascular surgery, neurosurgery, oncology surgery, and orthopedic surgery, delivering enhanced visualization of anatomical structures and pathological tissues. Imaging and diagnostics applications are also diversifying, extending from endoscopic fluorescence imaging to fluorescence microscopy, fluorescence-based ophthalmic imaging, and in vivo fluorescence techniques that enable noninvasive visualization at the molecular level. Beyond imaging, therapeutic applications are emerging that integrate photodynamic therapy and theranostic agents to treat cancerous lesions with simultaneous diagnostic monitoring.
On the End-User front, diagnostic laboratories continue to adopt fluorescence-based assays for high-throughput screening, hospitals and clinics are integrating imaging systems into operating rooms and outpatient procedures, and research and academic institutes are fueling innovation through preclinical studies and translational research. This comprehensive segmentation analysis offers critical insights into where investments, partnerships, and product development efforts can yield the greatest impact.
Decoding Regional Nuances and Growth Drivers in the Americas Europe Middle East Africa and Asia Pacific Fluorescence Medical Device Ecosystem
Regional performance in the fluorescence medical device sector is shaped by unique combinations of healthcare infrastructure, reimbursement policies, and innovation ecosystems. In the Americas, established markets in North America are driving demand for precision-guided therapies and portable diagnostic platforms, fueled by a strong network of academic medical centers and well-defined regulatory pathways. Latin America is beginning to follow suit, as public health initiatives and expanding healthcare coverage create opportunities for cost-effective imaging solutions.Across Europe, the Middle East, and Africa, diverse healthcare standards are giving rise to targeted adoption strategies. Western European countries are leading in early adoption of advanced fluorescence-guided interventions thanks to robust clinical trials and centralized reimbursement frameworks. Meanwhile, Middle Eastern and African regions are selectively investing in core applications where fluorescence can address pressing challenges such as infectious disease diagnostics and surgical safety, often through public-private partnerships and international collaborations.
In the Asia-Pacific region, rapid economic growth and large patient populations are driving significant investments in healthcare infrastructure. Countries with mature medical device industries are pioneering innovations in fluorescence microscopy and image-guided surgery, while emerging economies are prioritizing cost-efficient fluorescence diagnostics to improve access to early disease detection. Together, these diverse regional narratives underscore the importance of localized go-to-market strategies and tailored value propositions for each market.
Highlighting Leading Industry Players Steering the Fluorescence Medical Device Market through Strategic Innovations and Collaborative Partnerships
The competitive landscape of fluorescence medical devices is characterized by an ecosystem of specialized innovators and established medical technology companies. Leading players have been prioritizing research and development to expand their fluorescence portfolios, often through acquisitions of niche fluorophore developers and strategic partnerships with imaging hardware specialists. These alliances enable comprehensive solutions that integrate dye chemistry, optical design, and real-time analytics software.Notable organizations are also collaborating with academic research centers to validate new imaging methods and accelerate clinical adoption. By conducting multi-center trials, such companies are demonstrating the safety and efficacy of fluorescence-guided procedures across different patient populations. This collaborative model not only strengthens credibility with regulatory bodies but also cultivates a pipeline of data that informs future product enhancements.
In addition, forward-looking players are investing in digital platforms that interpret fluorescence signals through machine learning algorithms, thereby offering predictive insights and automated decision support. Such digital ecosystems are emerging as differentiators in a crowded market, enabling end users to extract more value from existing imaging hardware. As the sector matures, the ability to deliver integrated fluorescence solutions-combining advanced dyes, precision optics, and intelligent software-will define the next wave of market leadership.
Prescriptive Action Plans for Industry Leaders to Leverage Fluorescence Advancements for Enhanced Medical Device Development and Competitive Advantage
Industry leaders seeking to capitalize on the fluorescence medical device opportunity should adopt a proactive, cross-functional strategy that spans R&D, regulatory, and commercialization functions. First, aligning internal research efforts with emerging clinical use cases will ensure that product pipelines address genuine unmet needs. Engaging with key opinion leaders early in the development process can accelerate protocol design and strengthen the evidentiary framework necessary for regulatory approval.Second, forging strategic partnerships with specialty chemical manufacturers and software developers will help mitigate supply chain risks and unlock integrated platform capabilities. Co-development agreements for novel fluorophore formulations can secure exclusive access to unique optical signatures, while alliances with analytics firms can deliver next-generation image interpretation tools. These collaborations should be underpinned by clear intellectual property arrangements to protect proprietary innovations.
Finally, tailored market access plans are essential for navigating diverse reimbursement landscapes. By demonstrating clear value through health economics studies and real-world evidence, companies can expedite coverage decisions in multiple regions. Simultaneously, investing in training programs for clinicians and technicians will facilitate smooth adoption and maximize clinical impact. By executing these action plans, industry leaders can reinforce their competitive position and drive long-term growth.
Ensuring Rigorous Insights through a Robust Research Methodology Combining Multiple Data Sources and Analytical Frameworks
A robust research methodology lays the foundation for credible insights into the fluorescence medical device landscape. This study combines primary research through interviews with surgeons, radiologists, laboratory directors, and procurement specialists to capture real-world perspectives on clinical utility and purchasing drivers. Secondary research draws on peer-reviewed journals, regulatory databases, patent filings, and conference proceedings to track technological developments and competitive moves.Quantitative analysis of supplier landscapes and product pipelines is performed by cross-referencing public filings, clinical trial registries, and company websites. In parallel, an expert advisory group comprised of industry veterans and academic researchers provides ongoing validation of emerging trends and segmentation boundaries. This hybrid approach ensures that findings are grounded in data while benefiting from the nuanced understanding of experienced professionals.
Finally, scenario modeling is used to assess the impact of variables such as tariff changes, regulatory updates, and adoption curves for AI-driven imaging tools. By stress-testing key assumptions, the methodology highlights both risks and opportunities, enabling stakeholders to make informed strategic decisions. Together, these methods deliver a rigorous, multi-dimensional view of the fluorescence medical device market.
Summarizing Key Findings and Charting the Future Trajectory of Fluorescence Innovations in the Evolving Medical Device Landscape
The analysis of fluorescence medical devices reveals a sector poised for continued expansion and innovation. Key findings highlight the growing importance of integrated imaging platforms that combine multispectral fluorescence with advanced analytics, the strategic imperative of diversifying supply chains in light of new tariffs, and the value of targeted collaboration between dye chemists and technology providers. Regional insights underscore the need for customized approaches in mature and emerging markets alike, while segmentation analysis points to high-growth opportunities in surgical guidance and in vivo diagnostics.Looking forward, the convergence of fluorescence imaging with artificial intelligence and theranostic therapies will unlock new clinical applications, from real-time tumor margin assessment to personalized drug activation. Companies that successfully navigate regulatory complexities, secure resilient supply chains, and build comprehensive digital ecosystems will be best positioned to lead the market. Furthermore, the tailoring of reimbursement strategies and clinician training programs will be critical for driving adoption and demonstrating long-term value.
As the medical device landscape evolves, stakeholders must remain agile, continuously refining their portfolios and partnerships. By leveraging the insights presented in this report, decision-makers can anticipate next-generation trends and allocate resources to the most promising avenues. Ultimately, the successful integration of fluorescence technologies will redefine patient care standards and create sustainable competitive advantage.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Technology
- Fluorescence Imaging Systems
- Fluorescence Spectroscopy
- Fluorescent Dyes & Agents
- Fluorophore Type
- Endogenous Fluorophores
- Exogenous Fluorophores
- Application
- Fluorescence in Catheters
- Fluorescence-Guided Surgery
- Cardiovascular Surgery
- Neurosurgery
- Oncology Surgery
- Orthopedic Surgery
- Imaging & Diagnostics
- Endoscopic Fluorescence Imaging
- Fluorescence Microscopy
- Fluorescence-Based Ophthalmic Imaging
- In Vivo Fluorescence Imaging
- Therapeutic
- End-User
- Diagnostic Laboratories
- Hospitals & Clinics
- Research & Academic Institutes
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- ADVACAM s.r.o.
- Arthrex, Inc.
- Bruker Corporation
- Carl Zeiss AG.
- Getinge AB
- Hamamatsu Photonics K.K.
- KARL STORZ SE & Co. KG
- Leica Microsystems GmbH
- LI-COR Biotech, LLC
- Mauna Kea Technologies
- Medtronic International Trading Sárl
- MolecuLight Inc.
- Olympus Corporation of the Americas
- OnLume Inc.
- Optomedic.
- PerkinElmer U.S. LLC
- PicoQuant GmbH
- Shimadzu Corporation
- Stryker Corporation
- Xuzhou Hengjia Electronic Technology Co., Ltd.
- SurgiMab SAS
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Fluorescence in Medical Device market report include:- ADVACAM s.r.o.
- Arthrex, Inc.
- Bruker Corporation
- Carl Zeiss AG.
- Getinge AB
- Hamamatsu Photonics K.K.
- KARL STORZ SE & Co. KG
- Leica Microsystems GmbH
- LI-COR Biotech, LLC
- Mauna Kea Technologies
- Medtronic International Trading Sárl
- MolecuLight Inc.
- Olympus Corporation of the Americas
- OnLume Inc.
- Optomedic.
- PerkinElmer U.S. LLC
- PicoQuant GmbH
- Shimadzu Corporation
- Stryker Corporation
- Xuzhou Hengjia Electronic Technology Co., Ltd.
- SurgiMab SAS
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
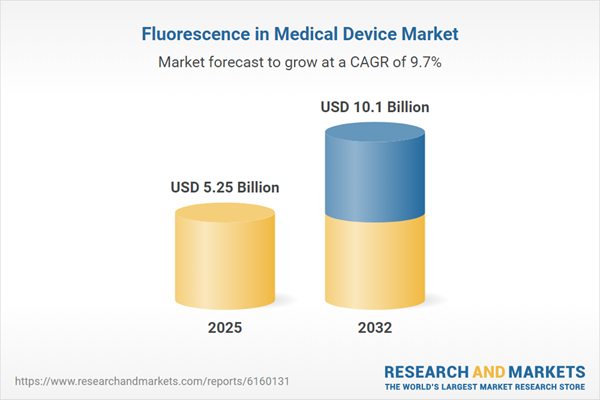
| Estimated Market Value ( USD | $ 5.25 Billion |
| Forecasted Market Value ( USD | $ 10.1 Billion |
| Compound Annual Growth Rate | 9.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 22 |