Speak directly to the analyst to clarify any post sales queries you may have.
Introducing the Pivotal Role of Solid State Catheters Bridging Precision Sensing Capabilities with Enhanced Patient Outcomes and Industrial Monitoring Advances
Solid state catheters have emerged as a cornerstone of precision sensing in both clinical environments and industrial process monitoring. This executive summary presents an integrated view of the key technological innovations performance trends and strategic factors influencing this dynamic field. By combining durability with miniaturization and real-time data capture capabilities, solid state catheters are enabling new diagnostic and interventional procedures that improve patient outcomes and operational efficiency.At the heart of this evolution lies the convergence of advanced materials microfabrication techniques and signal processing algorithms. These developments have led to significant gains in measurement accuracy sensitivity and reliability, addressing critical demands in cardiovascular neurology gastroenterology and beyond. As such, stakeholders from device manufacturers to healthcare providers and research institutes are adapting to a landscape defined by rapid innovation cycles and tighter regulatory scrutiny.
This summary will delve into transformative technological shifts the effects of recent United States tariffs supply chain complexities segmentation insights regional trends and leading industry players. It concludes with actionable recommendations to help industry leaders capitalize on emerging opportunities and navigate challenges effectively. By synthesizing the most relevant data and expert perspectives, this executive summary sets the stage for informed strategic decision making and sustainable growth in the solid state catheter domain.
Exploring the Rapid Technological Convergence That Is Reshaping Solid State Catheter Design Manufacturing and Clinical Integration Ecosystems
The landscape of solid state catheter design and application is undergoing a profound transformation catalyzed by rapid advances in microelectromechanical systems and integrated sensing technologies. As miniaturization becomes more sophisticated, manufacturers are embedding multiple sensing modalities within a single catheter platform, thereby expanding clinical applications and reducing the need for multiple devices during complex interventional procedures. This shift toward multifunctional platforms has been further accelerated by progress in advanced packaging techniques that ensure device robustness in harsh physiological and industrial environments.Moreover, the integration of wireless data transmission and cloud-based analytics has redefined expectations around real-time monitoring and remote diagnostics. Clinicians can now access continuous pressure flow and temperature data streams from implanted devices, enabling early detection of complications and proactive intervention strategies. In parallel, industrial operators leverage similar capabilities to optimize process control research and development workflows, ensuring consistent performance under variable operating conditions.
Furthermore, the industry’s embrace of AI-driven signal processing is enhancing the interpretability of high-volume sensor data. Algorithms trained on extensive datasets can identify subtle anomalies and predict device wear or patient complications before they manifest clinically. Consequently, the solid state catheter ecosystem is transitioning from reactive procedural support tools to proactive platforms that drive personalized care and predictive maintenance. This strategic convergence of materials science electronics and data analytics underscores the next era of innovation in the field.
Unpacking the Cumulative Effects of Newly Imposed United States Tariffs on Materials Components and Supply Chains Affecting Solid State Catheter Development
The imposition of new tariffs by the United States government in 2025 has introduced significant cost pressures and supply chain complexities for manufacturers of solid state catheters. Raw materials such as high-purity quartz ceramics and specialized silicon substrates now face increased import duties, compelling device producers to reconsider their sourcing strategies. Consequently, the anticipated cost advantages of certain piezoelectric and MEMS-based sensing components have eroded, driving some organizations to seek alternative supply partners or invest in domestic fabrication capabilities.In addition, tariffs on precision machining equipment and semiconductor packaging materials have affected the production timelines for both fiber optic and MEMS sensor assemblies. Companies reliant on imported interferometric and polarimetric fiber optic elements have experienced lead-time extensions, which in turn have disrupted project schedules and procurement plans. As a result, many firms are reevaluating their global manufacturing footprints and exploring nearshoring or reshoring initiatives to mitigate exposure to fluctuating trade policies.
Moreover, the cascading impact of these tariffs has extended to sterilization and assembly operations. Autoclave and gamma radiation facilities that depended on imported components have had to adjust pricing structures and absorb incremental costs or pass them along to end users. In the face of these challenges, some industry leaders have formed strategic alliances to pool sourcing volumes and share the burden of tariff-induced expenses. Ultimately, the 2025 tariff framework underscores the critical need for agile supply chain management and diversified sourcing and highlights the importance of ongoing policy monitoring for maintaining competitive resilience.
Illuminating Key Segmentation Insights Revealing How Type Application End User Material Technology Assembly and Sterilization Influence Market Dynamics
A nuanced understanding of the market requires close attention to the multiple dimensions that shape solid state catheter demand and innovation. Analysis by type reveals distinct value propositions: fiber optic variants, which include intensity based sensors, interferometric designs and polarimetric configurations, offer unparalleled immunity to electromagnetic interference and high sensitivity for pressure monitoring. MEMS-based catheters further diversify the landscape, with capacitive sensors optimizing low-pressure applications, diaphragm sensors balancing range and stability, and piezoresistive elements delivering robust performance under variable flow conditions. Meanwhile, piezoelectric types composed of ceramic and quartz materials provide exceptional frequency response characteristics crucial for capturing high-resolution dynamic pressure signals.When viewed through the lens of application, industrial use cases such as process control systems and research and development environments demand rugged reliability and extended operational cycles. Conversely, medical applications spanning cardiovascular interventions, gastrointestinal diagnostics, neurological monitoring and urological assessments require biocompatible materials and precise miniaturization for patient safety and procedural efficacy. End user segmentation further stratifies demand; diagnostic centers prioritize consistent throughput and device versatility, home healthcare settings emphasize ease of use and disposability, hospitals require comprehensive integration with clinical workflows, and research institutes seek customizable platforms for experimental protocols.
Material selection also plays a pivotal role. Ceramic, metal, polymer and silicon substrates each present tradeoffs in terms of biocompatibility flexibility, cost and manufacturability. Packaging technologies such as chip-on-board and chip-scale approaches enhance form factor and reliability, while flexible electronics enable conformable interfaces. Traditional packaging techniques remain relevant for applications demanding proven durability. In assembly considerations, disposable catheters reduce infection risk and streamline logistics, whereas reusable formats offer long-term cost efficiencies. Finally, sterilization methods-autoclave, ethylene oxide and gamma radiation-determine process flows and regulatory compliance, thereby influencing the total cost of ownership and clinical adoption rates.
Unveiling Critical Regional Trends and Growth Drivers Spanning the Americas Europe Middle East Africa and AsiaPacific in Solid State Catheter Adoption
Regional dynamics exert a profound influence on innovation trajectories and adoption rates within the solid state catheter market. In the Americas, strong collaboration between medical device clusters and leading academic institutions propels advancements in sensor technologies, while favorable reimbursement policies and well-established healthcare infrastructure support rapid clinical uptake. This environment also fosters industrial partnerships that drive process control applications in sectors ranging from petrochemicals to food and beverage, reinforcing the region’s dual focus on medical and industrial use cases.Moving to Europe, the Middle East and Africa, stringent regulatory frameworks and a diverse mix of emerging and mature markets shape adoption pathways. In Western Europe, high per-capita healthcare spending and initiatives promoting minimally invasive procedures accelerate demand for multifunctional catheter platforms. Conversely, emerging markets in the Middle East and Africa demonstrate growing interest in cost-effective sterilization methods, such as ethylene oxide and gamma radiation, to expand access to advanced diagnostic modalities while managing capital constraints.
Meanwhile, the Asia-Pacific region stands out for its rapidly expanding healthcare infrastructure and manufacturing capabilities. Domestic sensor fabrication hubs in East Asia are driving down component costs for fiber optic and MEMS-based catheters, enabling wider access across urban and rural clinical settings. Additionally, government investments in smart manufacturing and research collaborations are fueling innovations in flexible electronics and traditional packaging adaptations tailored to local market needs. Consequently, Asia-Pacific is poised to become a key driver of global solid state catheter deployment, with a strong focus on balancing affordability and performance.
Highlighting Leading Manufacturers Strategic Alliances and Innovations Driving Competitive Differentiation in the Solid State Catheter Ecosystem
The competitive environment is characterized by a blend of established medical device conglomerates and specialized sensor technology firms vying for leadership through innovation and strategic alliances. Leading participants have pursued partnerships with materials science laboratories to develop next-generation piezoelectric composites that enhance sensitivity while reducing footprint. Concurrently, select sensor manufacturers have integrated AI-enabled signal processing modules into their catheter platforms to differentiate their offerings by delivering predictive analytics and automated anomaly detection.In addition, several major players have undertaken targeted acquisitions to strengthen their end-to-end capabilities, encompassing fiber optic sensor production through to sterilization and assembly services. Such consolidations aim to streamline supply chains bolster intellectual property portfolios and accelerate time to market. Moreover, joint development agreements between medical OEMs and semiconductor fabricators are becoming increasingly common, reflecting a strategy to co-design packaging and interface solutions that optimize performance in specific clinical procedures.
Beyond M&A activity, investment in pilot manufacturing facilities and test laboratories underscores a commitment to quality assurance and regulatory compliance. Firms are collaborating with accredited research institutes to validate new sensor architectures under a range of physiological and industrial conditions, thereby ensuring that device performance aligns with both clinical standards and process control requirements. Taken together, these strategic moves illustrate how leading companies are differentiating through technological prowess operational scale and collaborative ecosystems to address diverse market needs.
Providing Actionable Strategic Recommendations to Industry Leaders Aiming to Optimize Innovation Investment and Accelerate Solid State Catheter Adoption
Industry leaders should prioritize cross-disciplinary R&D programs that integrate materials science, microfabrication and data analytics to accelerate the development of multifunctional catheter platforms. By allocating resources to pilot production and rapid prototyping facilities, organizations can validate emerging sensor designs under realistic operating conditions and refine manufacturing processes before full-scale deployment. In conjunction with these efforts, companies must strengthen their supply chain resilience by diversifying sourcing across multiple geographies and exploring nearshoring or domestic fabrication to mitigate trade policy disruptions.Furthermore, forging strategic partnerships with regulatory consultants and clinical research organizations will streamline approval pathways and support evidence generation for new device indications. This collaborative approach can shorten time to market and enhance clinical acceptance, particularly in high-growth therapeutic areas such as neurological monitoring and cardiovascular intervention. Simultaneously, engaging end users through iterative feedback loops-incorporating insights from diagnostic centers, hospitals and home healthcare providers-will ensure that product innovations align closely with real-world procedural workflows and patient needs.
To maximize market penetration in regions with distinct regulatory and cost structures, tailored commercialization strategies are essential. In mature markets, emphasizing premium performance attributes and integrated analytics will resonate with providers focused on advanced care. Conversely, in emerging markets, cost-optimized product variants and flexible sterilization partnerships can expand access while preserving device quality. Ultimately, a balanced portfolio approach underpinned by robust strategic planning and agile execution will position industry leaders to capture value across the diverse solid state catheter ecosystem.
Detailing the Rigorous Research Methodology Employed to Ensure Data Accuracy Comprehensive Analysis and Informed Solid State Catheter Market Insights
The research methodology underpinning this executive summary combines rigorous primary and secondary data gathering techniques. Initially, in-depth interviews were conducted with key stakeholders across the value chain, including device manufacturers, clinical end users and supply chain partners. These qualitative discussions yielded insights into emerging technological priorities, regulatory hurdles and purchasing criteria, which were then triangulated with publicly available patent filings, academic journal articles and regulatory databases.Subsequently, quantitative analyses of market segmentation, regional adoption patterns and tariff impacts were performed using a proprietary benchmarking framework. Data inputs were validated through multiple rounds of expert review to ensure accuracy and consistency. Special emphasis was placed on mapping the interplay between material science advancements and sensor performance metrics, as well as correlating sterilization method preferences with clinical workflows.
To further enhance reliability, the study incorporated scenario analysis addressing potential shifts in trade policy, supply chain disruptions and technological breakthroughs. These scenarios were stress-tested against historical industry performance and current innovation trajectories. Finally, all findings were synthesized in close collaboration with domain specialists in materials engineering, electronics packaging and healthcare economics, ensuring that the conclusions and recommendations reflect the collective wisdom of the most relevant experts in the solid state catheter field.
Concluding the Executive Summary with a Synthesis of Core Findings Industry Implications and Future Outlook for Solid State Catheter Advancements
In summary, the solid state catheter landscape is being reshaped by converging technological innovations, evolving regulatory frameworks and shifting trade dynamics. Precision sensing modalities-spanning fiber optic, MEMS and piezoelectric architectures-are unlocking new possibilities in cardiovascular gastroenterological neurological and urological diagnostics, while packaging and sterilization choices further refine device performance and clinical integration.Simultaneously, 2025 tariff adjustments have underscored the necessity for agile supply chain strategies and diversified sourcing, prompting manufacturers to reassess their global footprints and invest in domestic capabilities. Regional disparities in healthcare infrastructure and regulatory environments, from the Americas to Europe Middle East Africa and AsiaPacific, highlight the importance of tailored commercialization approaches that align product features with local needs and cost considerations.
Key industry players are responding through strategic alliances, M&A activity and targeted R&D collaborations designed to deliver differentiated catheter platforms with embedded analytics and predictive capabilities. For industry leaders navigating this complex ecosystem, a balanced focus on innovation, operational resilience and customer-centric design will be critical. By leveraging the insights and recommendations detailed in this summary, stakeholders can confidently chart a course toward sustainable growth and technological leadership in the solid state catheter arena.
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Type
- Electrophysiology Catheter
- Intravascular Ultrasound Catheter
- Pressure Monitoring Catheter
- Temperature Monitoring Catheter
- Material
- Ceramic
- Metal
- Polymer
- Usability Type
- Disposable
- Reusable
- Application
- Cardiology
- Gynecology
- Neurology
- Brain Perfusion Monitoring Catheter
- Intracranial Pressure Monitoring Catheter
- Urology
- End User
- Diagnostic Centers
- Hospitals & Clinics
- Research Institutes
- Distribution Channel
- Offline
- Direct Sale
- Distributor Network
- Online
- Offline
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- LABORIE MEDICAL TECHNOLOGIES CORP.
- Medtronic plc
- Millar, LLC
- EB Neuro S.p.A.
- Koninklijke Philips N.V.
- Blue Light Co., Ltd.
- Medline Industries, LP.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Solid State Catheter market report include:- LABORIE MEDICAL TECHNOLOGIES CORP.
- Medtronic plc
- Millar, LLC
- EB Neuro S.p.A.
- Koninklijke Philips N.V.
- Blue Light Co., Ltd.
- Medline Industries, LP.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | October 2025 |
| Forecast Period | 2025 - 2032 |
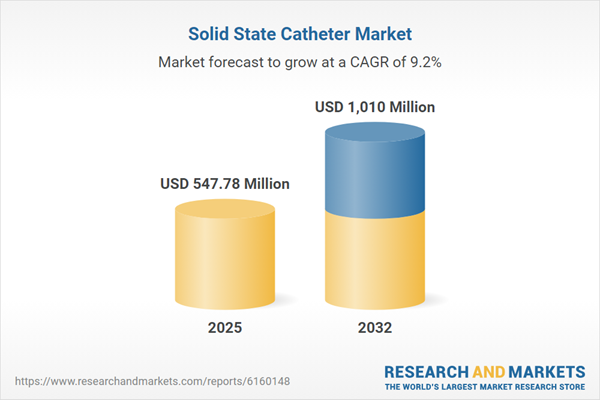
| Estimated Market Value ( USD | $ 547.78 Million |
| Forecasted Market Value ( USD | $ 1010 Million |
| Compound Annual Growth Rate | 9.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 8 |