Speak directly to the analyst to clarify any post sales queries you may have.
Evolving Innovations in Ophthalmic Surgery Instruments Driving Precision and Patient Outcomes Across Diverse Clinical and Technological Frontiers
In recent years, the ophthalmic surgery instruments sector has undergone an unprecedented wave of innovation that has fundamentally reshaped surgical protocols and patient outcomes. Precision engineering, driven by advancements in microfabrication and ergonomic design, has elevated the standard of care in procedures ranging from cataract removal to complex vitreoretinal interventions. Surgeons now rely on meticulously crafted blades, forceps, and motorized systems that enhance dexterity, reduce operative time, and minimize trauma to delicate ocular tissues.Simultaneously, the integration of digital technologies has accelerated the pace of transformation in the operating suite. High-resolution imaging platforms, real-time data analytics, and connectivity between surgical consoles and enterprise systems have enabled a seamless exchange of information, empowering clinicians to make informed decisions at critical junctures. This digital overlay not only supports precision but also fosters predictive maintenance of instrumentation, ensuring that equipment remains in optimal condition.
Moreover, stakeholder expectations have evolved alongside these technological breakthroughs. Healthcare providers increasingly seek instruments that offer not just functional reliability but also value-based outcomes, driving manufacturers to refine cost structures and adopt sustainable practices. As a result, disposable consumables are being engineered with eco-friendly materials, while reusable instruments are being redesigned for sterilization efficiency and longevity.
As demographic shifts lead to a rising incidence of age-related ocular conditions, the demand for specialized surgical tools continues to climb, underscoring the urgency for scalable solutions that meet both clinical efficacy and economic pragmatism.
Together, these dynamics set the stage for a market characterized by rapid innovation, heightened competition, and a relentless focus on improving patient safety and surgical success rates. Understanding the forces at play is essential for stakeholders aiming to navigate this complex landscape and seize emerging opportunities.
Emergence of Minimally Invasive Techniques and Digital Integration Redefining Surgical Protocols and Workflow Efficiencies in Ophthalmic Care
Minimally invasive surgical techniques have gained remarkable traction in recent years, fostering a paradigm where smaller incisions, microcatheter-based approaches, and refined instrumentation converge to reduce patient discomfort and accelerate postoperative recovery. In the realm of glaucoma and vitreoretinal surgery, for example, the shift toward sutureless ports and microincision strategies has compelled manufacturers to develop ultra-fine blades, specialized cannulas, and ergonomic handpieces that facilitate precise manipulation within confined anatomical spaces.In parallel, the confluence of digital integration and robotics is redefining the scope of what is possible in ophthalmic surgery. Advanced motorized systems now incorporate haptic feedback, allowing surgeons to experience tactile sensations that mimic direct tissue interaction. Coupled with real-time image guidance and augmented reality overlays, these platforms are ushering in an era of enhanced situational awareness, reducing the margin for error and elevating the consistency of outcomes. The integration of cloud-based analytics further empowers surgical teams to benchmark performance metrics, optimize workflow, and proactively address maintenance needs through predictive algorithms.
These transformative shifts are not confined to high-acuity hospital environments. Ambulatory surgery centers are rapidly adopting modular instrument carts and portable digital workstations, democratizing access to sophisticated surgical capabilities. As such, the market is witnessing a convergence of innovation, where cross-disciplinary collaboration between engineers, data scientists, and clinical experts is driving the next wave of high-performance, patient-centric solutions.
Moreover, the integration of telemedicine platforms for preoperative planning and postoperative monitoring is enabling remote support models, further enhancing the continuum of care and reducing patient burden. This relentless pursuit of technological synergy underscores the imperative for stakeholders to cultivate adaptive strategies that align with evolving clinical protocols and regulatory frameworks
Navigating the Implications of New United States Trade Tariffs on Supply Chains, Cost Structures and Competitive Dynamics within Ophthalmic Instrumentation
The introduction of new tariffs by the United States government has introduced a complex layer of considerations for manufacturers, distributors, and healthcare providers in the ophthalmic surgery instruments domain. Components ranging from precision stainless steel blades to titanium-handled forceps now bear the impact of revised duty structures, compelling stakeholders to reassess cost models and procurement strategies. For multinational players, these measures have prompted a reexamination of global supply chains, with a growing emphasis on nearshoring and regional assembly hubs to circumvent elevated import levies.Simultaneously, smaller specialized suppliers are exploring partnerships with domestic foundries to mitigate exposure to tariff volatility. Such collaborations seek to maintain material quality and production standards while streamlining logistics. However, transitioning manufacturing footprints is not without challenges; stringent regulatory approvals require thorough documentation and quality validation, which can extend time-to-market for essential instrumentation.
Healthcare providers are adjusting their procurement policies in response to incremental price adjustments. Ambulatory surgery centers and hospital systems are increasingly engaging in consortium purchasing agreements, leveraging collective bargaining power to negotiate better terms and stabilize supply. This trend underscores the rising importance of transparent pricing mechanisms and long-term supplier relationships in a landscape influenced by trade policy.
Building resilient supply chain architectures that incorporate multiple sourcing nodes and enhanced inventory management will be integral to sustaining operational continuity under changing tariff scenarios. Engagement in multilateral trade dialogues and leveraging frameworks such as USMCA can offer alternative pathways to stabilize import tariffs and foster cooperative standards across North American supply hubs.
Looking ahead, the capacity to navigate evolving tariff regimes will distinguish market leaders. By integrating flexible sourcing strategies, investing in regional infrastructure, and fostering partnerships across the value chain, stakeholders can mitigate cost pressures and maintain uninterrupted access to critical ophthalmic surgical instruments
Deep Analysis of Ophthalmic Instrument Market Segmentation Revealing How Procedure Types Product Categories Materials and End Users Shape Industry Trends
In analyzing the ophthalmic surgery instruments market, it is imperative to recognize the diverse procedural categories that drive demand. Cataract extraction remains a cornerstone procedure, supported by specialized phacoemulsification handpieces and microincision blades, while corneal surgeries require instruments tailored for tissue transplantation and refractive error correction. Procedures addressing glaucoma incorporate microcatheters and drainage devices, and oculoplastic interventions call for delicate scissors and forceps designed for periorbital tissue manipulation. Refractive surgeries depend on precision ablation tips and calibrated microkeratomes, and vitreoretinal operations hinge on high-performance cannulas, vitrectomy probes, and tubular instrumentation that navigate the posterior segment with minimal retinal disturbance.Product type segmentation reveals equally distinct considerations. Disposable consumables such as cannulas, tips, and tubing are engineered for sterility and single-use efficiency, reflecting a global push toward infection control. Manual instruments, including forceps, scissors, and speculums, prioritize ergonomic form factors and surgical precision, whereas motorized systems integrate advanced torque control and programmable settings to meet the exacting demands of complex microsurgeries. Reusable instruments, from surgical blades to precision handpieces, balance durability with straightforward sterilization protocols, ensuring long-term reliability.
Material composition further influences instrument performance and cost. Plastic components offer lightweight disposability, stainless steel delivers robust strength and corrosion resistance, and titanium provides a premium combination of biocompatibility and tensile integrity. End-user settings such as ambulatory surgery centers favor streamlined, cost-effective instrument portfolios, while hospitals and clinics require comprehensive inventories that support a broad spectrum of interventions. Distribution channels, whether facilitated through offline direct sales and distributor networks or via growing online platforms, shape accessibility and after-sales support, underscoring the multifaceted nature of segment-driven market dynamics
Uncovering Regional Dynamics in the Ophthalmic Surgical Instruments Landscape Illustrating Emerging Drivers and Growth Patterns Across Key Global Territories
Regional dynamics in the ophthalmic surgery instruments sphere reveal nuanced patterns driven by healthcare infrastructure, regulatory environments, and demographic trends. In the Americas, established surgical centers continue to adopt incremental innovations, with a strong emphasis on ambulatory care models and bundled payment frameworks. Manufacturers find fertile ground for introducing advanced motorized systems and premium reusable handpieces, leveraging well-developed distribution networks to reach both urban and suburban markets.Within Europe, the Middle East and Africa, the landscape is characterized by a diverse spectrum of market maturity. Western European nations emphasize patient-centric care pathways and stringent regulatory compliance, driving adoption of high-precision instruments and digital integration. Simultaneously, emerging economies in the Middle East are investing in state-of-the-art ophthalmic centers, spurring demand for specialized diagnostic tools and disposable consumables. In regions of Africa, mobile surgical programs and public-private partnerships are expanding access, creating opportunities for cost-effective manual instruments and robust sterilization solutions.
Across the Asia-Pacific, rapid urbanization and rising healthcare expenditure are catalyzing demand for both refractive and cataract surgery tools. High volumes of procedures in nations with large aging populations encourage local manufacturing collaborations and strategic alliances. Regulatory bodies in various countries are streamlining approval processes for new devices, facilitating quicker market entry. Online distribution channels are gaining traction, particularly in densely populated metropolitan areas, while traditional distributor networks continue to serve remote regions.
Collectively, these regional insights underscore the importance of tailored go-to-market strategies, localized partnerships, and adaptive regulatory navigation to capture growth in diverse economic and clinical settings
In-Depth Overview of Leading Ophthalmic Instrument Manufacturers Their Strategic Initiatives Technological Leadership and Partnerships Driving Industry Evolution
Leading manufacturers in the ophthalmic surgery instruments market have demonstrated strategic agility by investing in research and development, forming targeted alliances, and expanding their global footprints. For instance, one global leader has leveraged its expertise in advanced materials to introduce titanium-based handpieces that combine strength with ergonomic comfort as they navigate a broad spectrum of surgical procedures. Another prominent player has focused on modular motorized platforms that integrate seamlessly with imaging suites, offering surgeons real-time feedback and programmable performance presets tailored to specific clinical protocols.Collaborative ventures have become a hallmark of innovation, with companies partnering with academic institutions to co-develop laser-guidance systems and haptic feedback modules. These partnerships not only expedite product development cycles but also validate device efficacy through peer-reviewed clinical trials. In parallel, several established firms have acquired niche startups specializing in single-use items such as precision cannulas and microtubing, thereby broadening their disposable consumable portfolios to meet the growing emphasis on infection control and operational efficiency.
Regional expansion has also been a critical component of competitive strategy. By establishing localized manufacturing hubs and service centers, industry leaders can respond more quickly to regulatory changes and reduce lead times for instrument delivery. Additionally, investments in digital platforms that support remote diagnostics and predictive maintenance are enhancing after-sales support, strengthening customer loyalty, and creating new revenue streams.
Furthermore, leading firms are exploring artificial intelligence-driven calibration tools that optimize instrument performance, and digital health solutions that streamline surgical planning and remote diagnostics.
Strategic Roadmap for Industry Leaders Targeting Enhanced Collaboration Innovation and Adaptation to Regulatory and Market Shifts in Ophthalmic Surgical Field
To thrive in a landscape defined by rapid technological advancement and evolving trade policies, industry leaders should adopt a multifaceted strategic roadmap that prioritizes collaboration, innovation, and regulatory agility. First, forging cross-disciplinary partnerships that unite instrument designers, data scientists, and clinical specialists can catalyze the development of integrated solutions, such as smart motorized systems equipped with real-time analytics and intuitive user interfaces.Second, companies should invest in sustainable material alternatives and streamlined sterilization protocols to address both environmental imperatives and value-based purchasing criteria. Embracing circular economy principles-such as refurbishing reusable handpieces and recycling or repurposing plastic consumables-can yield cost efficiencies and reinforce brand reputation among environmentally conscious stakeholders.
Third, diversification of manufacturing footprints through regional assembly and nearshore production can mitigate exposure to tariff-related disruptions and geopolitical risk. By aligning production nodes with key end-user markets, organizations can shorten supply chains, reduce transportation costs, and accelerate regulatory approvals tailored to local standards.
Fourth, a proactive approach to regulatory forecasting is essential. Establishing dedicated teams to monitor policy shifts, engage with regulatory bodies, and participate in industry consortia ensures that product pipelines remain aligned with evolving compliance requirements.
Lastly, with online distribution channels gaining momentum, leaders should develop robust digital platforms that facilitate direct engagement with healthcare providers. Offering virtual training modules, predictive maintenance alerts, and performance benchmarking dashboards can enhance customer loyalty and create scalable service models that complement traditional sales channels
Comprehensive Research Methodology Combining Primary Expert Interviews Rigorous Secondary Data Analysis and Robust Validation Processes for Market Insights
This research effort employed a rigorous and systematic approach to capture the multidimensional dynamics of the ophthalmic surgery instruments market. Primary research formed the backbone of data collection, with in-depth interviews conducted with a diverse array of stakeholders, including ophthalmic surgeons, operating room managers, procurement specialists, and senior executives from leading instrument manufacturers. These discussions provided qualitative insights into clinical preferences, procurement challenges, and innovation priorities.Complementing primary inputs, secondary research encompassed the thorough review of peer-reviewed journals, regulatory filings, technical white papers, and industry publications. A comprehensive analysis of patent records and product launch announcements also contributed to understanding emerging technologies and competitive positioning.
Data validation processes were integral to ensuring accuracy and robustness. Triangulation of interview findings with secondary data points enabled the identification of consistent trends and the resolution of any discrepancies. In addition, a proprietary framework for assessing technological readiness and market adoption rates was applied to evaluate the diffusion of key innovations across geographic regions and end-user segments.
Finally, iterative reviews with subject-matter experts and advisory panels provided further scrutiny of preliminary findings, refining the final insights and recommendations. This multi-layered methodology ensures that the conclusions drawn are grounded in empirical evidence and reflective of current industry realities
Final Synthesis of Critical Learnings and Strategic Imperatives Shaping the Future Trajectory of Ophthalmic Surgical Instrumentation and Industry Competitiveness
In synthesizing the insights gathered from technological advances, trade policy impacts, segmentation dynamics, regional nuances, and competitive strategies, it becomes clear that the ophthalmic surgery instruments market is positioned at a pivotal inflection point. The convergence of minimally invasive techniques, digital integration, and material innovation is reshaping surgical protocols, enabling clinicians to achieve superior precision and patient outcomes. At the same time, macroeconomic factors such as revised tariff regimes are prompting stakeholders to reevaluate supply chain configurations and embrace regional manufacturing to maintain cost competitiveness.The segmentation analysis underscores the heterogeneity of market drivers, from procedural categories like cataract and vitreoretinal surgeries to product typologies spanning disposable consumables, motorized systems, and reusable handpieces. Materials such as stainless steel and titanium continue to underpin device reliability, while distribution channels evolve to balance offline direct sales networks with the convenience of online procurement.
Regionally, differentiated growth patterns in the Americas, Europe, Middle East & Africa, and Asia-Pacific highlight the need for localized strategies that address unique regulatory landscapes and end-user requirements. Leadership in this environment will favor organizations that combine strategic partnerships, sustainable practices, and agile regulatory foresight.
Looking forward, the intersection of advanced materials, automation, and data-driven decision support will continue to redefine surgical boundaries, presenting both challenges and opportunities for stakeholders committed to advancing ocular health worldwide
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Procedure Type
- Cataract
- Corneal
- Glaucoma
- Oculoplastic
- Refractive
- Vitreoretinal
- Product Type
- Disposable Consumables
- Cannulas
- Tips
- Tubing
- Manual Instruments
- Forceps
- Scissors
- Speculums
- Motorized Systems
- Reusable Instruments
- Blades
- Handpieces
- Disposable Consumables
- Material Type
- Plastic
- Stainless Steel
- Titanium
- End User
- Ambulatory Surgery Centers
- Hospitals & Clinics
- Distribution Channel
- Offline
- Direct Sale
- Distributor Network
- Online
- Offline
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Bausch+Lomb Incorporated
- Beaver-Visitec International
- Carl Zeiss AG
- Duckworth & Kent Ltd.
- GE Healthcare
- Geuder AG
- Halma PLC
- HOYA Corporation
- Johnson & Johnson Services Inc.
- Mani, Inc.
- Medtronic plc
- Nidek Inc.
- Oertli Instrumente AG
- Rumex International Co.
- Sklar Surgical Instruments, Inc.
- Sontec Instruments, Inc.
- SurgiEdge Corporation
- Surgitrac Instruments (UK) Ltd.
- Topcon Corporation
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Ophthalmic Surgery Instruments market report include:- Bausch+Lomb Incorporated
- Beaver-Visitec International
- Carl Zeiss AG
- Duckworth & Kent Ltd.
- GE Healthcare
- Geuder AG
- Halma PLC
- HOYA Corporation
- Johnson & Johnson Services Inc.
- Mani, Inc.
- Medtronic plc
- Nidek Inc.
- Oertli Instrumente AG
- Rumex International Co.
- Sklar Surgical Instruments, Inc.
- Sontec Instruments, Inc.
- SurgiEdge Corporation
- Surgitrac Instruments (UK) Ltd.
- Topcon Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 198 |
| Published | November 2025 |
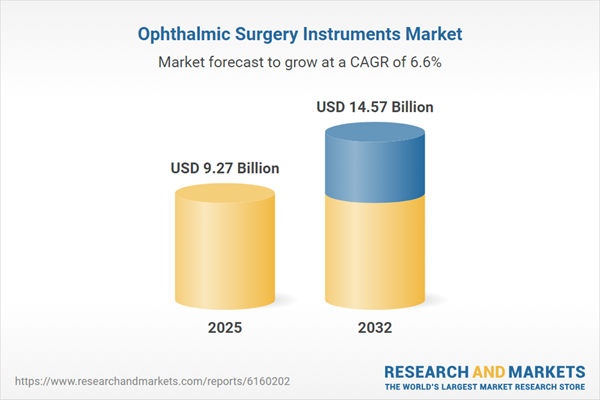
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 9.27 Billion |
| Forecasted Market Value ( USD | $ 14.57 Billion |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |