Speak directly to the analyst to clarify any post sales queries you may have.
Exploring the Essential Role and Market Dynamics of Luer Adapters in Modern Healthcare Delivery Systems to Drive Precision and Safety in Clinical Practice
Luer adapters serve as fundamental connectors in fluidic systems across diverse healthcare environments, ensuring precise and leak-free connections between syringes, tubing, and medical devices. These small yet critical components underpin countless clinical procedures ranging from routine blood sample collection to complex therapeutic infusions. As the demand for minimally invasive interventions and point-of-care diagnostics intensifies, luer adapters have emerged as indispensable elements in modern medical practice.Over the past decade, evolving treatment paradigms and the growth of outpatient care settings have driven renewed focus on connector reliability, biocompatibility, and ease of use. Innovations in polymer processing, stringent regulatory scrutiny, and the shift toward single-use disposables have collectively transformed how manufacturers design, validate, and market luer adapters. Consequently, stakeholders across the value chain-including component suppliers, device assemblers, and healthcare providers-are actively evaluating new materials, production techniques, and quality assurance protocols to enhance patient safety and operational efficiency.
Looking ahead, the luer adapter landscape will be shaped by the convergence of emerging technologies, increasing cost pressures, and global supply chain realignment. The introduction of smart connectivity features, alternative materials with improved environmental footprints, and heightened focus on standardized compatibility presents both challenges and opportunities for device makers, procurement teams, and regulatory bodies. As this report will demonstrate, a nuanced understanding of market dynamics, technology adoption curves, and evolving end-user requirements is essential for driving sustainable growth and ensuring optimal clinical outcomes.
Unveiling the Transformative Shifts Reshaping Luer Adapter Innovation Adoption and Regulatory Compliance Across Expanding Healthcare Supply Chains
The luer adapter sector has undergone profound transformations over recent years, driven by technological breakthroughs, shifting regulatory landscapes, and evolving clinical workflows. Fueled by the proliferation of point-of-care testing and ambulatory care models, manufacturers have prioritized the integration of barcoding, RFID tagging, and traceability mechanisms into connector designs. This trend toward enhanced device intelligence is complemented by growing emphasis on interoperability standards to minimize the risk of misconnections and bolster patient safety.Simultaneously, regulatory bodies worldwide have tightened oversight of fluidic connectors, implementing more rigorous validation criteria for leakage testing, material biocompatibility, and sterilization efficacy. In parallel, sustainability concerns have elevated the importance of recyclable and bio-sourced polymers, prompting R&D teams to explore resin blends that meet both performance requirements and environmental goals. As a result, product development pipelines now reflect a delicate balance between cost containment, eco-friendliness, and uncompromised clinical performance.
Moreover, the industry's competitive landscape has expanded as niche players introduce differentiated offerings-such as low-particle connectors tailored to sensitive laboratory applications-while established corporations invest in strategic partnerships and acquisitions to broaden their product portfolios. Taken together, these transformative shifts underscore the imperative for stakeholders to remain agile, informed, and proactive in addressing emerging challenges across manufacturing, supply chain resilience, and regulatory compliance.
Analyzing the Cumulative Impact of United States Tariffs Initiated in 2025 on Luer Adapter Supply Chains Manufacturing Costs and Pricing Strategies
The imposition of new United States tariffs in 2025 has exerted significant influence on the economics of luer adapter production and distribution. Prior to this policy change, a substantial portion of connector components and raw materials-particularly specialized polymers and precision-machined metal parts-were imported under preferential trade terms. The recalibration of duties has prompted device manufacturers to reexamine sourcing strategies and reassess total landed costs across their supply chains.In response to tariff pressures, many stakeholders have accelerated efforts to localize production, partnering with domestic injection-molding firms and precision machine shops to mitigate exposure to import levies. While this approach has yielded greater supply chain resilience, it has also necessitated upfront capital investments in tooling, quality assurance frameworks, and regulatory registrations. Some organizations have elected to offset higher unit costs by optimizing manufacturing yields through lean methodologies and advanced process controls.
On the buyer side, healthcare providers and group purchasing organizations have become more vigilant in negotiating long-term contracts and volume-based rebates to stabilize pricing. Simultaneously, emerging second-source suppliers in North America have gained traction by highlighting shorter lead times and collaborative product development models. Against this backdrop, the 2025 tariff landscape has catalyzed both operational realignment and strategic innovation, compelling industry participants to adopt more integrated, cost-effective, and flexible sourcing solutions.
Delivering Key Segmentation Insights Across Product Types Materials End Users Applications and Connector Configurations for Luer Adapter Markets
A detailed examination of product segmentation reveals that the market for luer adapters is structured around two primary connection mechanisms. Luer lock variants dominate applications where secure, threaded engagement is paramount-such as in infusion therapy and high-pressure diagnostic equipment-while luer slip configurations remain preferred for rapid, low-force attachments in point-of-care sampling and short-duration procedures.Material composition further delineates market dynamics, with metal adapters prized for their robustness, sterilization compatibility, and longevity. Brass components deliver consistent performance in demanding clinical environments, whereas stainless steel models provide superior corrosion resistance. Conversely, plastic connectors have surged in adoption due to their cost efficiency and single-use convenience. Polycarbonate formulations offer high clarity and dimensional stability for precision applications, polyethylene presents excellent chemical resistance for pharmaceutical transfer, and polypropylene stands out for its balanced mechanical properties and low extractables.
End-user segmentation underscores the multifaceted demand patterns across care settings. Ambulatory surgical centers and clinics prioritize convenience and inventory optimization, whereas diagnostic laboratories-spanning both medical and research facilities-require connectors engineered to stringent analytical tolerances. In home care environments, ease of use and minimal maintenance drive purchasing decisions, while hospitals-comprising both private and public institutions-demand scalable solutions that align with complex sterilization protocols and patient throughput requirements.
Application-based insights highlight diagnostic connectors designed for sample integrity, infusion adapters engineered for controlled flow rates, pharmaceutical connectors compliant with drug transfer standards, and transfusion-specific fittings that guarantee strict leak testing and compatibility. Finally, connector configuration shapes functionality and workflow integration, with straight adapters facilitating straightforward inline fluid transfer, cross fittings enabling multi-directional routing, T-shaped connectors supporting splitting or merging lines, and Y-shaped designs accommodating synchronous dual-infusion setups with minimal dead volume.
Comprehensive Regional Insights Highlighting Demand Drivers Supply Dynamics and Emerging Opportunities in Americas EMEA and Asia Pacific for Luer Adapters
Regional dynamics for luer adapters showcase a diverse set of growth drivers and market considerations. Throughout the Americas, the United States remains a hub for advanced medical device innovation, supported by robust regulatory frameworks and high per-capita healthcare spending. Canada's procurement policies emphasize safety and standardization, while several Latin American nations are modernizing hospital infrastructure and seeking cost-efficient single-use connectors to address infection control objectives.In Europe Middle East and Africa, regulatory harmonization across the European Union has facilitated faster product approvals and streamlined compliance for advanced material connectors. The United Kingdom, Germany, and France lead in research collaborations exploring biodegradable connector materials. Simultaneously, Gulf Cooperation Council countries are investing in domestic manufacturing capabilities to reduce import dependency, and select African markets are upgrading laboratory networks to support growing diagnostic volumes, driving demand for reliable luer fittings.
Asia-Pacific presents a landscape of contrasting maturity levels, with Japan and South Korea emphasizing precision manufacturing and sensor-integrated connectors for next-generation diagnostics. China's rapidly expanding biotech sector and government-backed initiatives to localize medical device production have spurred numerous joint ventures and technology transfers. India and Southeast Asia continue to focus on cost optimization and local validation pathways, while Australia and New Zealand prioritize high-quality disposable connectors aligned with stringent biocompatibility standards. Together, these regional variations underscore the importance of tailored strategies for product adaptation, regulatory engagement, and supply chain optimization.
Key Companies Shaping the Future of Luer Adapters Through Strategic Partnerships Innovation Portfolios and Competitive Positioning in Global Markets
Leading corporations in the luer adapter domain are distinguished by their robust innovation pipelines, strategic alliances, and broad manufacturing footprints. Large multinational medical device companies leverage integrated design-to-production capabilities to deliver standardized solutions at scale, often bundling connectors within complete infusion sets and analytic consumables. In contrast, specialized connectors manufacturers have carved out niches by focusing on advanced materials research or bespoke configurations for high-precision laboratory workflows.Collaborations between polymer technology firms and medical device OEMs have yielded proprietary resin formulations that reduce particulate contamination and enhance sterilization performance. At the same time, cross-industry partnerships-spanning electronics, software, and fluidics-are fostering the emergence of “smart connectors” capable of logging usage metrics and interfacing with digital health platforms. Competitive positioning is further reinforced through regional manufacturing hubs that shorten lead times and enable faster regulatory submissions.
Mergers and acquisitions continue to reshape the market hierarchy, as established entities seek to absorb complementary product lines and expand into emerging geographies. Meanwhile, lean market entrants target underserved segments by offering rapid prototyping services, small-batch runs, and direct engagement models with clinical end users. Collectively, these strategic moves illustrate a sector in which agility, technological differentiation, and global reach are critical determinants of sustainable success.
Actionable Recommendations for Industry Leaders to Enhance Luer Adapter Production Efficiency Regulatory Compliance and Market Penetration Strategies
Industry leaders should prioritize strategic diversification of their manufacturing bases, investing in both onshore and near-shore facilities to mitigate geopolitical uncertainties and tariff impacts. By adopting modular production platforms, companies can more rapidly scale output and introduce incremental design enhancements without extensive retooling. Concurrently, allocating R&D resources toward advanced polymer blends and additive manufacturing techniques will support the development of lighter, stronger, and more sustainable connector options.To reinforce market penetration, executives are encouraged to forge collaborative partnerships with healthcare providers and research institutions, co-creating tailored solutions that address specific clinical workflows and regulatory requirements. Integrating traceability features and digital interfacing capabilities will not only enhance product differentiation but also align with broader healthcare digital transformation initiatives. Furthermore, implementing rigorous data analytics frameworks across quality control processes will expedite root cause analysis and continuous improvement efforts.
Finally, embedding environmental stewardship within corporate strategies by pursuing circular economy principles-such as recyclable materials, take-back programs, and carbon footprint reporting-will resonate with value-based procurement mandates. Combined, these actionable recommendations will enable organizations to bolster resilience, accelerate innovation cycles, and secure leadership positions in the evolving luer adapter landscape.
Rigorous Research Methodology Detailing Primary and Secondary Data Collection Validation Techniques and Analytical Frameworks Underpinning the Luer Adapter Report
This report's findings are underpinned by a comprehensive research methodology that synthesizes primary interviews, secondary data sources, and rigorous analytical procedures. Primary research involved in-depth conversations with senior executives, R&D directors, procurement managers, and clinical end users across key geographies, ensuring first-hand perspectives on technology adoption drivers, quality expectations, and regulatory hurdles.Secondary research complemented these insights through systematic reviews of peer-reviewed journals, regulatory databases, patent filings, and industry white papers. Data triangulation techniques were applied to reconcile disparate information streams, bolstering the reliability of segmentation outcomes and trend analyses. All data points underwent validation against publicly disclosed financial reports, press releases, and trade association metrics to confirm accuracy and consistency.
Quantitative analyses employed statistical modeling to identify correlations between material choices, production methods, and performance metrics, while qualitative frameworks-such as scenario planning and SWOT assessments-enabled robust evaluation of competitive dynamics. Expert validation panels reviewed draft interpretations to refine key assumptions and ensure that all conclusions reflect real-world operational contexts. This multi-layered approach guarantees that the strategic insights presented herestand on a foundation of actionable intelligence and rigorous evidence gathering.
Concluding Perspectives on Luer Adapter Market Evolution Technological Integration and Strategic Imperatives for Sustained Growth and Clinical Excellence
The evolution of the luer adapter market reflects a confluence of technological innovation, regulatory advancement, and shifting healthcare delivery models. As the industry navigates the impacts of new tariff regimes, material sustainability imperatives, and rising demand for connectivity, stakeholders must remain vigilant in adapting their strategies. Enhanced segmentation insights and regional dynamics underscore the importance of targeted product development and supply chain agility.Leading organizations will differentiate themselves through investments in advanced materials, smart connectivity features, and collaborative partnerships that drive end-user value and reinforce patient safety. Simultaneously, companies that streamline their manufacturing footprint and integrate data-driven quality controls will better withstand market pressures and capitalize on emerging opportunities in diagnostics, infusion, and beyond. In an environment defined by rapid change, this report equips decision-makers with the insights and frameworks needed to chart a course toward sustainable growth and clinical excellence.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Quick‑Connect Luer Adapters
- Universal Luer Adapters
- Material
- Metal
- Brass
- Stainless Steel
- Plastic
- Polycarbonate
- Polyethylene
- Polypropylene
- Metal
- Connector Configuration
- Cross
- Straight
- T Shape
- Y Shape
- Application
- Diagnostics
- Infusion
- Pharmaceutical
- Transfusion
- End User
- Ambulatory Surgical Centers
- Clinics
- Diagnostic Laboratories
- Medical Laboratories
- Research Laboratories
- Home Care Settings
- Hospitals
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Amsino International Inc.
- B. Braun Melsungen AG
- Baxter International Inc
- Becton, Dickinson and Company
- Chemglass Life Sciences LLC
- CODAN Companies
- Eldon James Corp.
- ESI Technologies Group
- Gerresheimer AG
- Hamilton Company
- ICU Medical, Inc.
- Industrial Specialties Mfg. Inc.
- LILY MEDICAL CORPORATION
- Nipro Europe Group Companies
- Nordson Medical
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Luer Adapter market report include:- Amsino International Inc.
- B. Braun Melsungen AG
- Baxter International Inc
- Becton, Dickinson and Company
- Chemglass Life Sciences LLC
- CODAN Companies
- Eldon James Corp.
- ESI Technologies Group
- Gerresheimer AG
- Hamilton Company
- ICU Medical, Inc.
- Industrial Specialties Mfg. Inc.
- LILY MEDICAL CORPORATION
- Nipro Europe Group Companies
- Nordson Medical
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
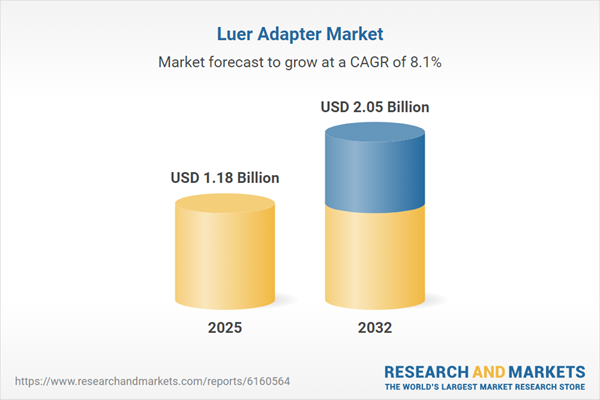
| Estimated Market Value ( USD | $ 1.18 Billion |
| Forecasted Market Value ( USD | $ 2.05 Billion |
| Compound Annual Growth Rate | 8.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |