Speak directly to the analyst to clarify any post sales queries you may have.
Exploring the Pivotal Role of Precision Craniotomy Instrument Kits in Optimizing Surgical Performance and Shaping the Future of Neurosurgical Care
Craniotomy instrument kits have emerged as indispensable components underpinning advanced neurosurgical interventions worldwide. These kits encompass a carefully curated ensemble of cutting and drilling instruments, retractors, and accessories tailored to the unique demands of cranial surgery. As neurosurgical procedures become increasingly intricate, the precision, ergonomic design, and reliability of each instrument within these kits directly influence procedural outcomes and patient safety. Moreover, the integration of innovative materials and engineering advances has elevated the functional performance of these tools, enabling surgeons to navigate complex anatomical challenges with greater confidence.Within this evolving clinical landscape, the adoption of powered drill systems alongside traditional manual instruments highlights a fundamental shift toward enhanced procedural efficiency. Concurrently, the emphasis on modular configurations has empowered surgical teams to customize kit contents in real time, aligning with specific case requirements and minimizing instrument fatigue. These developments not only streamline operating room workflows but also contribute to optimized instrument sterilization protocols and inventory management strategies.
As demographic shifts lead to an aging population and rising incidence of neurological conditions, the demand for craniotomy procedures has intensified. This trend underscores the necessity for instrument kits that balance cost-effectiveness with uncompromising quality standards. Partnerships between medical device innovators and clinical experts have further accelerated the refinement of instrument ergonomics, reducing surgeon fatigue and improving intraoperative precision. By contextualizing these drivers within the broader healthcare framework, this summary sets the stage for a deeper examination of transformative shifts, tariff implications, segmentation analysis, regional dynamics, and strategic recommendations that follow
Identifying the Transformational Forces and Technological Disruptions Redefining the Craniotomy Instrument Kits Market Landscape Across Modern Healthcare
Neurosurgical practice is undergoing a profound metamorphosis driven by converging technological innovations and policy reforms. Digital integration and the advent of smart instruments capable of interfacing with surgical navigation systems are at the forefront of this transformation. These breakthroughs have enabled real-time feedback on tool positioning and bone density, thereby enhancing surgical precision and reducing intraoperative risks. At the same time, materials science breakthroughs have yielded lightweight, corrosion-resistant alloys and polymers that extend instrument lifespan while maintaining sterilization efficacy.In parallel, regulatory bodies have catalyzed change through revised device classification frameworks and accelerated pathways for breakthrough medical technologies. Such regulatory evolutions have incentivized manufacturers to streamline product development cycles and prioritize compliance through rigorous quality management systems. Accordingly, market entrants are pursuing strategic collaborations with academic institutions and clinical centers to validate instrument performance in diverse surgical settings.
Equally significant is the shift toward minimally invasive and endoscopic approaches within neurosurgery, which has reshaped the design parameters of craniotomy kits. Manufacturers are now focusing on the miniaturization of drills and retractors without compromising durability or torque output. Furthermore, growing emphasis on environmental sustainability has spurred initiatives to reduce single-use instrument waste, leading to the emergence of reusable or multi-function tool sets. These collective shifts are redefining the competitive landscape and setting new benchmarks for clinical excellence
Assessing the Multilayered Implications of 2025 United States Tariffs on Supply Chain Economics and Strategic Pricing of Craniotomy Instrument Kits
In 2025, new United States tariff measures targeting imported medical devices have introduced significant complexity to the global supply chain for craniotomy instrument kits. These tariffs, which apply incremental duties on key components sourced from major manufacturing hubs, have rippled through procurement channels, compelling industry stakeholders to reassess sourcing strategies and cost structures. As a result, margins on imported kits have contracted, driving some distributors to renegotiate supplier contracts or absorb a portion of the increased costs to maintain competitiveness.Accordingly, device manufacturers and hospital procurement teams are exploring nearshoring opportunities to mitigate the impact of tariffs. Domestic production facilities that adhere to stringent quality standards are experiencing renewed investment interest, driven by the desire to secure uninterrupted supply lines and manage price volatility. Nevertheless, establishing or expanding local manufacturing capabilities entails capital-intensive processes and regulatory hurdles, underscoring the importance of a balanced approach that leverages both domestic and international sourcing.
Moreover, the tariff environment has prompted stakeholders to optimize inventory planning and implement dynamic pricing models. By adopting advanced analytics and demand forecasting tools, organizations can align purchase volumes with anticipated tariff schedules, thus minimizing exposure to sudden cost escalations. Transparency in cost allocation and close collaboration with clinical teams are integral to preserving budgetary predictability without compromising the quality or availability of critical surgical instruments
Decoding the Multidimensional Segmentation Landscape of Craniotomy Instrument Kits Through End Users Technologies Instrument Types and Applications
In examining the market through an end user lens, one finds that ambulatory surgical centers are increasingly outfitted with streamlined instrument kits optimized for high-volume outpatient procedures, where rapid turnaround and sterilization efficiency are paramount. Hospitals, encompassing both private institutions that prioritize cutting-edge innovation and public facilities focused on cost containment, demand kits that balance advanced functionality with budget considerations. Moreover, specialty clinics catering to niche neurosurgical subfields require bespoke configurations that accommodate unique procedural workflows, underscoring the importance of modularity in kit design.From a technology standpoint, manual kits continue to hold relevance for their simplicity, ease of sterilization, and lower upfront investment, particularly in resource-constrained environments. In contrast, powered kits have gained traction for their superior torque control and consistent performance under demanding surgical conditions. Within the powered category, electric systems offer precise speed modulation and seamless integration with navigation platforms, while pneumatic variants deliver lightweight portability and reduced maintenance overhead, thus appealing to diverse clinical preferences.
Instrument type analysis further refines the segmentation narrative by highlighting the distinct roles of cutting instruments, which encompass curettes, rongeurs, and scalpel handles designed for meticulous bone removal, and drill instruments subdivided into high speed drills that facilitate rapid cranial access and low speed drills suited for delicate structural adjustments. Retractor instruments, spanning handheld retractors that offer tactile responsiveness and self retaining retractors that maintain constant exposure, address varying surgeon ergonomics. When applied to clinical contexts such as neurotrauma interventions, pediatric cranial procedures, skull base operations, tumor resection protocols, and vascular surgery tasks, these instrument categories reveal nuanced demand patterns that inform both product development and procurement strategy
Unlocking Regional Dynamics and Market Drivers Shaping Demand for Craniotomy Instrument Kits Across the Americas Europe Middle East Africa and Asia Pacific
In the Americas, the United States stands at the forefront of neurosurgical innovation, supported by robust reimbursement frameworks and extensive clinical research networks. Private healthcare providers and academic medical centers are early adopters of next generation craniotomy instrument kits, fueling technology penetration. Canada’s stable regulatory environment and growing focus on minimally invasive surgery further bolster kit demand, while Latin American markets are gradually increasing procurement volumes as public health initiatives expand access to specialized neurosurgical care.Across Europe, Middle East & Africa, mature healthcare systems in Western Europe drive demand for advanced surgical tools, with regulatory harmonization under European directives ensuring consistent quality standards. Meanwhile, investment in healthcare infrastructure in the Gulf region and North African hubs has catalyzed the establishment of specialized neurosurgery centers. Local manufacturing partnerships and government funded projects are enhancing regional supply chain resilience, promoting wider accessibility of premium craniotomy kits.
Asia Pacific exhibits dynamic growth underpinned by increasing healthcare expenditure, rising incidence of neurological disorders, and the proliferation of medical tourism. China has emerged as a critical production base and consumer market, propelled by supportive government policies and domestic innovation. India’s expanding network of multispecialty hospitals is adopting both manual and powered instrument kits to address diverse surgical needs. Japan’s emphasis on precision engineering and quality assurance complements the broader regional trend toward sophisticated neurosurgical solutions
Profiling Leading Innovators and Competitive Strategies Driving Excellence and Market Expansion in the Craniotomy Instrument Kits Industry
Industry leaders in the craniotomy instrument kits realm consistently allocate significant resources to research and development, aiming to refine instrument ergonomics, integrate digital feedback mechanisms, and leverage advanced biomaterials for enhanced durability. These innovators collaborate with neurosurgeons and academic institutions to validate new prototypes, ensuring that emerging solutions address clinical pain points during instrument navigation, bone consistency evaluation, and tissue retraction. Their robust intellectual property portfolios and compliance with international quality standards reinforce market credibility and foster sustained portfolio expansion.In parallel, competitive strategies center on strategic alliances and targeted acquisitions that extend geographic reach and broaden product offerings. Several prominent providers have forged partnerships with software developers to introduce instrument management platforms that optimize inventory control and track usage metrics in real time. Others have established specialized training academies and simulation centers to educate surgical teams, thereby strengthening brand loyalty and accelerating technology adoption. By differentiating through customer service excellence, customizable kit configurations, and comprehensive after sales support, these leading companies are effectively positioning themselves at the nexus of innovation and clinical utility
Formulating Actionable Strategic Recommendations to Propel Growth Operational Efficiency and Competitive Advantage in Craniotomy Instrument Kits Market
To navigate the evolving landscape of craniotomy instrument kits, industry participants should consider establishing or expanding domestic manufacturing capabilities to reduce exposure to tariff fluctuations and enhance supply chain resilience. By forming joint ventures with local partners and leveraging contract manufacturing organizations, device producers can accelerate time to market without compromising on stringent quality controls. Additionally, integrating dynamic pricing models and advanced analytics for demand forecasting will enable procurement teams to optimize inventory levels and maintain cost predictability.Furthermore, manufacturers and healthcare providers ought to invest in modular kit architectures and smart instrument technologies that support seamless integration with surgical navigation systems and data analytics platforms. Emphasizing sustainability through reusable instrument components and eco friendly sterilization processes not only aligns with global environmental mandates but also reduces long term operational expenses. Collaborative training initiatives, including simulation based education and digital skill development programs, will cultivate greater familiarity with advanced toolsets among surgical teams, driving adoption rates. Finally, forging cross sector partnerships with software developers and academic research centers can spur innovation pipelines and unlock new avenues for product differentiation, thereby fortifying competitive advantage
Elucidating the Rigorous Research Framework and Analytical Methodologies Underpinning the Comprehensive Craniotomy Instrument Kits Market Study
This study employed a robust research framework combining primary and secondary methodologies to deliver a comprehensive understanding of the craniotomy instrument kits market. Primary data was gathered through structured interviews with neurosurgeons, purchasing managers, and device company executives, capturing firsthand insights on clinical preferences, procurement challenges, and emerging technology needs. These interactions were complemented by site visits to leading surgical centers, where observational data on instrument utilization and sterilization protocols enriched the qualitative analysis.Secondary research included an exhaustive review of peer reviewed journals, industry white papers, regulatory filings, and patents to contextualize technological trends and compliance landscapes. Market segmentation was validated through data triangulation techniques, cross referencing published import export statistics, product catalogs, and financial disclosures. Quantitative analysis employed time series assessments to identify historical patterns, while scenario modeling was used to evaluate potential impacts of policy shifts such as tariff implementations. Finally, all findings were subjected to expert panel review for accuracy and relevance, ensuring that strategic recommendations are grounded in empirically sound evidence and reflective of current industry dynamics
Synthesizing Key Executive Takeaways and Strategic Insights to Navigate the Craniotomy Instrument Kits Landscape with Confidence and Agility
In summary, the craniotomy instrument kits market is characterized by dynamic technological advancements, shifting regulatory frameworks, and evolving supply chain considerations. Precision powered instruments and smart connectivity are redefining surgical standards, while segmentation across end user categories, instrument types, and applications underscores the importance of tailored kit solutions. The imposition of 2025 US tariffs has introduced cost pressures that necessitate agile sourcing strategies and investments in nearshoring to safeguard supply continuity.Regional nuances-from the innovation hubs in North America and Western Europe to the burgeoning demand in Asia Pacific and the Middle East-offer distinct opportunities for market participants to align product offerings with local clinical needs. Leading companies that marry modular design, digital integration, and sustainability initiatives will be best positioned to capture value. By adopting the strategic recommendations outlined herein, stakeholders can enhance operational efficiency, strengthen competitive positioning, and ultimately deliver superior patient outcomes in the field of neurosurgery
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Instrument Type
- Drills & Burrs
- Elevators & Curettes
- Forceps
- Retractors
- Scalpels & Blades
- Suction Devices
- Material Type
- Carbon Fiber
- Plastic/Polymer
- Stainless Steel
- Titanium
- Packaging Type
- Reusable Instrument Kits
- Single-Use Disposable Kits
- End User
- Academic & Research Institutions
- Ambulatory Surgical Centers
- Hospitals
- Private Hospitals
- Public Hospitals
- Specialty Neurosurgery Clinics
- Application
- Aneurysm Clipping
- Hydrocephalus Treatment
- Endoscopic Third Ventriculostomy
- Shunt Placement
- Trauma Management
- Hemorrhage Control
- Skull Fracture Fixation
- Tumor Resection
- Glioma
- Meningioma
- Pituitary Tumors
- Distribution Channel
- Offline
- Direct Sales
- Distributors
- Authorized Distributors
- Third-party Distributors
- Specialty Stores
- Online
- Company Websites
- E-commerce Platforms
- Offline
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- B. Braun Melsungen AG
- Stryker Corporation
- Medtronic plc
- Integra LifeSciences Corporation
- Zimmer Biomet Holdings, Inc.
- KLS Martin Group
- Adeor Medical AG
- Johnson & Johnson Services, Inc.
- Olympus Corporation
- Richard WOLF
- Shanghai Medical Instruments
- Boston Scientific Corporation
- Teleflex Incorporated
- CooperSurgical
- ConMed Corporation
- Hubly Surgical Inc
- IRRAS AB
- Mizuho America Inc
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Craniotomy Instrument Kits market report include:- B. Braun Melsungen AG
- Stryker Corporation
- Medtronic plc
- Integra LifeSciences Corporation
- Zimmer Biomet Holdings, Inc.
- KLS Martin Group
- Adeor Medical AG
- Johnson & Johnson Services, Inc.
- Olympus Corporation
- Richard WOLF
- Shanghai Medical Instruments
- Boston Scientific Corporation
- Teleflex Incorporated
- CooperSurgical
- ConMed Corporation
- Hubly Surgical Inc
- IRRAS AB
- Mizuho America Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
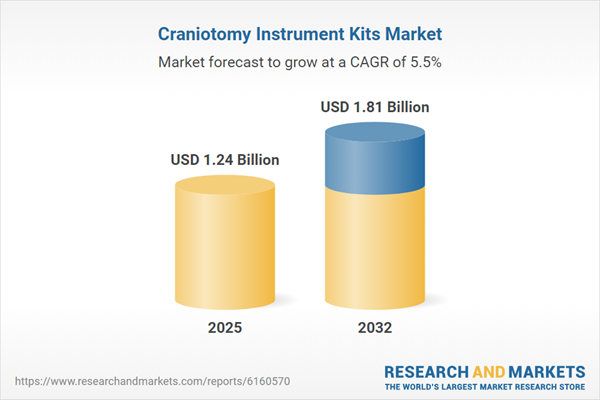
| Estimated Market Value ( USD | $ 1.24 Billion |
| Forecasted Market Value ( USD | $ 1.81 Billion |
| Compound Annual Growth Rate | 5.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 19 |