Speak directly to the analyst to clarify any post sales queries you may have.
A focused orientation to clinical presentation, care complexities, and stakeholder priorities that define contemporary approaches to disruptive mood dysregulation disorder
Disruptive mood dysregulation disorder (DMDD) occupies a complex intersection between pediatric psychiatry, developmental psychology, and systems of care that are still adapting to rising clinical and social demand. Clinicians and health systems face the dual challenge of differentiating chronic irritability and severe temper outbursts from comorbid conditions, while also identifying evidence-based interventions that are appropriate for younger patients. The diagnostic clarity introduced over the past decade has improved recognition, yet persistent gaps remain in standardizing care pathways across diverse clinical settings.Consequently, treatment decisions often balance psychopharmacology, psychotherapy, family-focused approaches, and educational supports. Those decisions are made within constrained operational environments where inpatient capacity, outpatient accessibility, and clinician bandwidth vary significantly. As a result, payers, hospitals, specialty clinics, and digital health providers are re-evaluating how to coordinate multidisciplinary care. New technologies and service models are entering a field historically driven by face-to-face therapeutic relationships, which requires careful evaluation of efficacy, safety, and equity in access.
This executive summary synthesizes current clinical trends, regulatory and reimbursement shifts, delivery model innovations, and strategic imperatives for stakeholders committed to improving outcomes for children and adolescents with disruptive mood dysregulation. Through a concise lens, the following sections articulate the forces reshaping care, the segmentation that matters for product and service design, and the regional and commercial dynamics that will influence near-term decisions and long-term planning.
An overview of the converging clinical, technological, policy and care-delivery forces that are redefining therapeutic approaches and access for pediatric mood disorders
The landscape for managing pediatric disruptive mood dysregulation is undergoing notable transformation driven by converging clinical, technological, and policy forces. Clinically, refined diagnostic criteria and growing awareness among primary care providers have increased referrals to specialists, prompting a reassessment of treatment algorithms and a shift toward early intervention strategies. At the same time, evolving evidence has clarified where psychotherapy, parent-directed interventions, and selective pharmacotherapies can be most effective, thereby influencing clinical guidelines and care pathways.Technological adoption is altering access and continuity of care. Telehealth platforms have expanded the reach of specialized behavioral interventions into previously underserved communities, and digital therapeutics are beginning to augment traditional cognitive behavioral therapy. In parallel, data analytics and remote monitoring tools are enabling more precise symptom tracking and outcome measurement, which supports personalized care plans and more timely adjustments to therapy. These shifts are prompting payers and health systems to consider novel reimbursement models that reward outcomes and continuity rather than episodic visits.
Regulatory attention and policy initiatives focused on pediatric mental health have increased funding and programmatic support for integrated school-based services and community mental health infrastructure. Meanwhile, manufacturers and service providers are adapting commercial strategies to prioritize long-term engagement, combination offerings, and cross-sector partnerships with education and social services. Taken together, these transformative shifts are redefining expectations for clinical efficacy, operational scalability, and measurable impact across patient cohorts.
A synthesis of how tariff adjustments enacted in 2025 are reshaping supply resilience, procurement economics, and clinical access across pediatric therapeutic pathways
The policy action in 2025 introducing adjusted tariff measures in the United States has created a cascade of effects across supply chains, procurement practices, and therapy accessibility without changing the underlying clinical need. First, the import-dependent components of pharmaceutical supply and medical devices-particularly those tied to parenteral formulations and ancillary packaging-have faced higher landed costs and administrative friction. Manufacturers and distributors responded by reassessing supplier portfolios and adjusting inventory strategies in order to secure continuity of treatment, which has had operational implications for hospitals, specialty clinics, and pharmacy networks.Because route of administration considerations shape clinical choices, any strain on the availability or cost of injectables, including prefilled syringe formats and vial supplies, can nudge prescribers and institutions toward oral therapies where clinically appropriate. Conversely, when clinical indications favor injectables, the constrained supply dynamics have increased the importance of vendor relationships and onshore manufacturing options. These supply-side shifts have also affected distribution channels: hospital pharmacies and specialty clinics have had to tighten purchasing cycles, while online and retail pharmacies restructured sourcing and fulfillment to preserve reliability for families and clinicians.
Beyond immediate logistics, tariff-driven adjustments have influenced investment priorities. Manufacturers that previously relied on cross-border sourcing are now accelerating local production planning and strategic supplier diversification. Research institutes and clinical trial operations reassessed material procurement timelines to prevent interruptions to pediatric studies. In aggregate, the tariff changes in 2025 have amplified the need for resilience-focused planning across stakeholders, encouraging stronger collaboration between clinical, commercial, and supply-chain teams to mitigate access disruptions.
Practical segmentation insights connecting therapeutic modality, care setting, administration route, age cohort, distribution pathway and end-user roles to actionable design decisions
A clear understanding of segmentation dynamics provides practical guidance for product development and service design. When viewed through therapeutic modalities, treatment landscapes encompass pharmacologic classes such as antidepressants, antipsychotics, mood stabilizers, and stimulants alongside behavioral approaches including cognitive behavioral therapy and parent training programs; each modality carries distinct evidence profiles, safety considerations, and implementation requirements that influence clinician adoption and payer scrutiny. Care settings further modulate those choices, with inpatient environments prioritizing acute stabilization and multidisciplinary coordination while outpatient settings focus on longitudinal management, access, and adherence supports.Route of administration is a practical determinant of logistics and patient preference: oral formulations tend to be easier to deliver in community settings, whereas injectable options-whether configured as vials or prefilled syringes-are selected when clinical response or formulation stability necessitates parenteral delivery. Age segmentation creates another layer of differentiation; interventions must be tailored to developmental considerations and family involvement, with adolescents (12-17 years) often engaging differently in therapy and medication management compared with children (6-11 years). Distribution channels shape the final mile of access, as hospital pharmacies, retail outlets, specialty clinics, and online pharmacies each offer distinct inventory models, patient counseling capabilities, and fulfillment timelines.
Finally, end-user categories anchor commercialization and service strategies: digital health consumers seek convenience and remote engagement, hospitals and mental health clinics require integrated care pathways and procurement reliability, research institutes prioritize standardized measures and protocol integrity, and schools and educational institutions are key partners for early identification and community-based interventions. Integrating these segmentation lenses enables stakeholders to design interventions that align clinical appropriateness with delivery feasibility and reimbursement realities.
Regional analysis that illuminates how care models, reimbursement priorities and digital adoption patterns across major territories influence access and strategy
Regional dynamics frame access, reimbursement, and service innovation in ways that are both complementary and distinct. In the Americas, there is broad momentum toward integrating behavioral health into primary care networks, and payers are experimenting with value-oriented contracts and outcome tracking that reward continuity and demonstrated improvement. This environment supports rapid deployment of telehealth and digital tools, but it also exposes disparities in rural and socioeconomically disadvantaged populations that require targeted outreach and school-based programs to bridge access gaps.Across Europe, Middle East & Africa, health systems demonstrate diverse maturity levels in pediatric mental health provision. Some European jurisdictions emphasize guideline-driven care and centralized specialist services, while parts of the Middle East and Africa are focused on capacity building, workforce training, and scaling community mental health initiatives. These differences create opportunities for collaboration on training, evidence dissemination, and scalable parent-focused interventions, and they underscore the need for adaptable delivery models that reflect local healthcare infrastructures.
In the Asia-Pacific region, rapid digital adoption and a growing emphasis on mental health literacy have catalyzed innovative service models, from school-linked screening programs to app-supported therapeutic platforms. However, regulatory heterogeneity and variable reimbursement mechanisms mean that cross-border commercialization requires careful localization of clinical content, supply arrangements, and stakeholder engagement strategies. Overall, regional nuances influence where to prioritize partnerships, how to structure distribution agreements, and which evidence packages will resonate with payers and providers in each jurisdiction.
Strategic corporate behaviors, partnership models and evidence-generation priorities that are shaping competitive positioning and long-term clinical adoption
Companies operating in this space are deploying a range of strategies to differentiate offerings and deepen clinical impact. Pharmaceutical and biologics developers emphasize pediatric-friendly formulations, safety data generation, and label-appropriate messaging in order to build clinician confidence. At the same time, firms with behavioral products are investing in evidence generation and interoperability to integrate digital tools with clinician workflows. Strategic partnerships between therapeutic manufacturers and digital platform providers are becoming more common, as firms seek to combine pharmacologic treatment with adherence support, symptom tracking, and caregiver education to improve real-world outcomes.Commercial organizations are also evolving distribution strategies by strengthening relationships with hospital systems, specialty clinics, and pharmacy networks to ensure reliable supply and point-of-care education. Some companies are piloting on-site training programs for school and community providers to facilitate early detection and referral. On the R&D front, sponsors are prioritizing pediatric-centric trial designs, adaptive protocols, and real-world evidence collection to accelerate demonstration of safety and functional outcomes. Collectively, these behaviors underscore a market orientation that favors integrated, multi-channel approaches and long-term stakeholder engagement rather than single-product promotional tactics.
Actionable strategic moves for leaders to align research, supply resilience, stakeholder engagement and commercialization with evolving clinical and policy priorities
Industry leaders should prioritize an integrated approach that aligns clinical development with delivery system realities and stakeholder needs. Investments in pediatric-suited formulations and combination care models that pair pharmacologic treatments with scalable behavioral interventions will increase clinical relevance and payer acceptance. At the same time, strengthening relationships with hospital systems, specialty clinics, schools, and digital partners can create reliable referral pathways and improve continuity of care for young patients.Operationally, building supply chain resilience through supplier diversification, contingency inventory, and consideration of localized manufacturing options will mitigate the kinds of procurement risks highlighted by tariff-driven disruptions. From a commercial perspective, developing clear value narratives supported by real-world outcome measures and caregiver-reported endpoints will facilitate conversations with payers and health systems. Finally, proactive engagement with regulatory agencies, professional societies, and advocacy groups will help shape favorable policy environments and support broader adoption of evidence-based practices. Taken together, these actions create a coordinated blueprint for advancing access, improving outcomes, and sustaining programmatic scalability.
A transparent methodological overview detailing evidence synthesis, expert validation, real-world data triangulation and limitations to ensure analytic rigor
The research underpinning these insights relied on a structured, multi-method approach designed to ensure validity, triangulation, and contextual relevance. Primary inputs included qualitative interviews with practicing child and adolescent psychiatrists, pediatricians, behavioral therapists, hospital pharmacists, clinic administrators, and payer representatives to capture real-world operational constraints and clinical decision drivers. Secondary evidence synthesis reviewed clinical guidelines, peer-reviewed literature, trial registries, and policy statements to ground interpretations in the most current scientific and regulatory thinking.Quantitative analyses leveraged de-identified utilization datasets and claims-based patterns where available, supplemented by prescription trend observations and supply chain indicators to identify operational bottlenecks. Throughout the process, findings were cross-validated with advisory panels composed of clinical and payer experts to refine assumptions, interpret ambiguous signals, and prioritize implications. The methodology also incorporated sensitivity checking and explicit documentation of limitations, including the variable availability of pediatric-specific outcome measures and the heterogeneity of regional reimbursement practices. This rigorous approach ensures that conclusions reflect a careful synthesis of evidence and practitioner insight, enabling practical decision support.
A concise summation integrating clinical, policy, supply chain and commercial imperatives to guide near-term priorities and long-term strategic planning
In sum, the care environment for disruptive mood dysregulation disorder is at a strategic inflection point. Enhanced diagnostic clarity and growing clinical recognition have increased the imperative to deliver evidence-informed, developmentally appropriate interventions across multiple settings. Technological advances and alternative delivery models offer clear opportunities to improve access and continuity, but they must be integrated with proven behavioral and pharmacologic approaches in order to achieve meaningful outcomes. Additionally, policy and procurement dynamics, exemplified by recent tariff-driven supply considerations, highlight the importance of resilience and adaptive commercial strategies.Moving forward, stakeholders who align clinical innovation with robust supply arrangements, rigorous outcome measurement, and cross-sector partnerships will be best positioned to address unmet needs. The synthesis presented here aims to focus attention on practical levers-formulation design, care pathway integration, digital augmentation, and stakeholder engagement-that can accelerate improvements in care delivery. Ultimately, a coordinated, data-informed approach that centers the needs of children, adolescents, and their caregivers will produce the most sustainable advances in treatment access and clinical outcomes.
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Treatment Type
- Antidepressants
- Antipsychotics
- Cognitive Behavioral Therapy (CBT)
- Mood Stabilizers
- Parent Training Programs
- Stimulants
- Care Setting
- Inpatient
- Outpatient
- Route Of Administration
- Injectable
- Prefilled Syringe
- Vial
- Oral
- Injectable
- Age Group
- Adolescents (12-17 years)
- Children (6-11 years)
- Distribution Channel
- Hospital Pharmacies
- Online Pharmacies
- Retail Pharmacies
- Specialty Clinics
- End User
- Digital Health Consumers
- Hospitals
- Mental Health Clinics
- Research Institutes
- Schools & Educational Institutions
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Johnson & Johnson Services, Inc.
- Eli Lilly and Company
- Otsuka Pharmaceutical Development & Commercialization, Inc.
- Pfizer Inc.
- Novartis AG
- Dr. Reddy’s Laboratories Ltd.
- Cerata Pharmaceuticals LLP
- Maya Biotech Pvt. Ltd.
- Ajanta Pharma Inc.
- Synnat Pharma Private Limited
- Biesterfeld Plastic GmbH
- Cipla Limited
- Aurobindo Pharma Inc.
- Zydus Lifesciences Limited.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Disruptive Mood Dysregulation Disorder market report include:- Johnson & Johnson Services, Inc.
- Eli Lilly and Company
- Otsuka Pharmaceutical Development & Commercialization, Inc.
- Pfizer Inc.
- Novartis AG
- Dr. Reddy’s Laboratories Ltd.
- Cerata Pharmaceuticals LLP
- Maya Biotech Pvt. Ltd.
- Ajanta Pharma Inc.
- Synnat Pharma Private Limited
- Biesterfeld Plastic GmbH
- Cipla Limited
- Aurobindo Pharma Inc.
- Zydus Lifesciences Limited.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | October 2025 |
| Forecast Period | 2025 - 2032 |
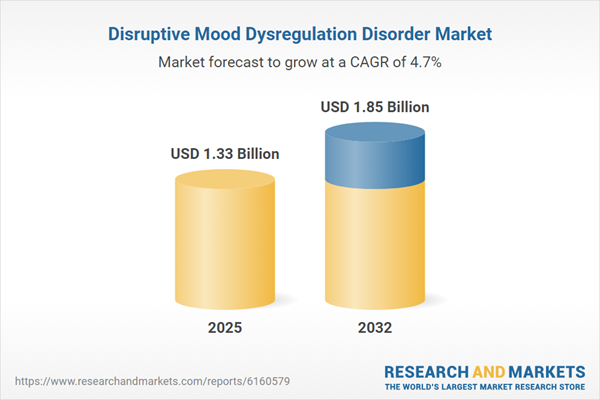
| Estimated Market Value ( USD | $ 1.33 Billion |
| Forecasted Market Value ( USD | $ 1.85 Billion |
| Compound Annual Growth Rate | 4.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |