Speak directly to the analyst to clarify any post sales queries you may have.
Setting the Stage for the Future of Inhalational Anesthesia Equipment Through Innovation, Safety Standards, and Market Evolution
Inhalational anesthesia equipment has long been a cornerstone of modern surgical practice, providing controlled delivery of anesthetic gases to patients undergoing complex interventions. Over the decades, these systems have progressed from rudimentary vaporizer assemblies to sophisticated consoles that integrate precision vaporizers, advanced breathing circuits, and real-time respiratory gas monitoring. As surgical procedures grow in complexity and patient expectations for safety and comfort increase, the importance of reliable, accurate, and user-friendly anesthesia hardware has never been greater.Today, clinicians and hospital administrators demand equipment that not only delivers consistent anesthetic concentrations but also streamlines workflow, enhances patient safety, and supports compliance with stringent regulatory standards. Continuous innovation has led to the adoption of digital controls, computer-assisted dosing algorithms, and portable configurations suitable for a wide variety of clinical settings. Moreover, the convergence of electronic, mechanical, and pneumatic technologies has opened new avenues for improving ventilation performance, reducing anesthetic gas wastage, and enabling remote monitoring capabilities.
This report begins by presenting a clear overview of the key factors shaping the inhalational anesthesia equipment landscape. It then explores the transformative shifts impacting product design, regulatory challenges posed by emerging tariffs, and the critical segmentation insights that inform strategic decision-making. By contextualizing these developments within the broader healthcare environment, this analysis aims to equip decision-makers with the knowledge required to navigate an increasingly dynamic market.
Exploring the Transformative Shifts Reshaping Inhalational Anesthesia with Digital Integration, Sustainability Focus, and Patient Centric Care Models
Recent years have witnessed a profound transformation in the way inhalational anesthesia equipment is designed and implemented, driven principally by advances in digital capabilities and a growing emphasis on sustainable healthcare practices. Innovations such as computer-controlled anesthesia machines have enabled more precise dosing through automated algorithms, minimizing human error while optimizing gas consumption. In parallel, integrated respiratory gas monitors equipped with advanced sensors provide clinicians with continuous feedback on end-tidal concentrations, improving safety margins and reducing the likelihood of under- or overdosing.Sustainability has emerged as another pivotal driver, with industry stakeholders exploring eco-friendly anesthetic agents and systems engineered to curtail greenhouse gas emissions. Engineers are refining vaporizers and breathing circuits to limit anesthetic gas leakage, while design teams are experimenting with lightweight, reusable materials to reduce disposable waste. As environmental concerns intersect with cost constraints, the impetus to adopt greener solutions has never been more compelling.
Moreover, patient-centric care models are reshaping equipment requirements, with healthcare providers seeking portable anesthesia systems that accommodate ambulatory surgical centers and remote clinics. Closed and semi-closed system architectures have gained traction for their capacity to deliver consistent results in varied clinical environments. Ultimately, these transformative shifts are converging to redefine standards for patient safety, operational efficiency, and environmental stewardship across the inhalational anesthesia ecosystem.
In addition to technological and environmental drivers, evolving regulatory frameworks and reimbursement models are shaping equipment adoption. Healthcare authorities are increasingly linking reimbursement rates to procedural efficiency and patient outcomes, incentivizing investments in machines that support anesthesia depth monitoring and integrated data reporting. Payers and hospital networks are evaluating controlled substance management features that reduce waste and demonstrate cost savings, further reinforcing the business case for next-generation anesthesia consoles.
Analyzing the Cumulative Impact of Landmark United States Tariffs Set for 2025 on Inhalational Anesthesia Equipment Supply Chains and Pricing Dynamics
The introduction of new United States tariffs in 2025 marks a significant inflection point for the inhalational anesthesia equipment market, introducing a complex array of cost and supply chain considerations. Components such as precision valves, microprocessor modules, specialized sensors, and mechanical flow controllers may be subject to increased duties, raising production costs for manufacturers and potentially triggering higher price points for end users. In turn, healthcare providers may experience tighter budgetary constraints as they seek to balance quality of care with cost-effective procurement.Beyond immediate pricing pressures, these tariffs have compelled many global suppliers to reevaluate their sourcing strategies. Manufacturers are now exploring alternative supply bases, including localizing production lines or forging strategic partnerships with domestic fabricators to mitigate dependence on high-tariff imports. Concurrently, procurement teams within hospitals and clinics are assessing consortium purchasing models and long-term supply agreements to lock in favorable terms and minimize exposure to ongoing tariff volatility.
Looking ahead, the cumulative impact of these trade measures is expected to accelerate efforts toward supply chain resilience and spur innovation in cost optimization. Firms that proactively adapt their manufacturing footprints and establish diversified distribution networks will be better positioned to navigate the evolving landscape, maintain competitive pricing, and ensure uninterrupted access to critical inhalational anesthesia solutions.
Furthermore, the tariff regime is prompting shifts in corporate procurement strategies, with budgetary allocations increasingly directed toward inventory buffering and distributor partnerships that assume greater pricing risk. Suppliers are enhancing after-sales service offerings to retain customer loyalty in an environment where capital expenditures may be constrained. These adaptations will influence long-term relationships across the value chain and accelerate the evolution of leasing and managed equipment services as alternative financing models.
Unlocking Key Insights from Product Type, System Type, Technology, End User, and Application Segmentation to Navigate Inhalational Anesthesia Equipment Dynamics
A nuanced understanding of market segmentation is essential for stakeholders aiming to tailor their offerings and identify high-potential opportunities within the inhalational anesthesia equipment landscape. When considering product type, the ecosystem encompasses anesthesia machines, breathing circuits, respiratory gas monitors, and vaporizers. Within the anesthesia machines category, distinctions emerge between computer-controlled systems designed for automated precision, continuous flow machines optimized for stable gas delivery, and portable units that cater to point-of-care flexibility. Breathing circuits vary in configuration to support different patient populations, while respiratory gas monitors deliver critical data for real-time adjustments. Vaporizers round out the portfolio by providing controlled drug delivery, with designs tuned for compatibility with specific anesthetic agents.From the perspective of system type, differentiation between closed and semi-closed configurations and open systems underscores trade-offs in gas conservation versus workflow simplicity. Medical facilities prioritize closed pathways to reduce waste, whereas open approaches may be favored for their ease of use in emergency settings. In terms of technology, electronic innovations have introduced touchscreen interfaces and digital diagnostics, mechanical solutions continue to deliver robust performance under challenging conditions, and pneumatic designs remain valued for their reliability in resource-constrained environments.
End user considerations further refine market opportunities, with ambulatory surgical centers and clinics seeking streamlined, cost-effective platforms, and hospitals requiring comprehensive capabilities for high-volume procedures. Research institutes place a premium on modular systems that support experimental protocols. Finally, application areas range from dental procedures and emergency medicine to pain management and surgical specialties such as cardiovascular, neurosurgery, and orthopedics, with veterinary use expanding alongside rising demand for pet healthcare. By aligning strategies with these interconnected segments, enterprises can more effectively meet the specific needs of diverse stakeholders and accelerate adoption across multiple clinical contexts.
Regional Trends Unveiled Across Americas Europe Middle East & Africa and Asia Pacific Revealing Growth Opportunities in Inhalational Anesthesia Equipment
Across the Americas, robust investments in healthcare infrastructure and the expansion of ambulatory surgical centers have driven demand for advanced inhalational anesthesia systems. North American markets are characterized by a willingness to adopt cutting-edge technologies such as network-enabled anesthesia machines and integrated respiratory gas monitoring solutions. In South America, rising government spending on healthcare has created new avenues for equipment suppliers, particularly in urban centers where facility upgrades are underway to support minimally invasive and outpatient procedures.In Europe, Middle East & Africa, regulatory harmonization efforts and stringent safety standards have elevated expectations for system reliability and compliance. Western European nations have embraced eco-conscious practices, leading to the deployment of low-emission vaporizers and reusable breathing circuits. Meanwhile, in the Middle East, rapid hospital modernization and medical tourism initiatives are fueling demand for versatile anesthesia platforms capable of serving diverse patient profiles. Sub-Saharan African regions present unique challenges related to power stability and resource availability, prompting interest in pneumatic and mechanically driven systems that ensure consistent performance.
The Asia-Pacific realm exhibits dynamic growth underpinned by population-scale healthcare needs and government-sponsored facility enhancements. Countries such as China and India are transitioning from basic anesthesia delivery hardware toward more sophisticated machines equipped with digital interfaces and telemonitoring capabilities. Southeast Asian markets are emerging as fertile grounds for portable anesthesia solutions in remote clinics and disaster relief scenarios. Collectively, these regional trends highlight the importance of nuanced market entry strategies and the tailoring of product portfolios to local clinical, regulatory, and environmental conditions.
Dissecting the Strategies and Innovations of Leading Corporations Driving the Evolution of Inhalational Anesthesia Equipment in a Competitive Market
Leading players in the inhalational anesthesia equipment arena are forging distinct pathways to maintain technological leadership and expand their global footprint. Major medical device firms have prioritized research and development investments to advance features such as automated dosing algorithms, cloud-enabled data analytics, and ergonomic design enhancements. These initiatives are complemented by strategic collaborations with academic institutions and technology startups to accelerate innovation cycles and anticipate clinician requirements.In pursuit of geographic expansion, several well-established corporations have entered into joint ventures and distribution agreements that facilitate access to underpenetrated markets. By leveraging local partners' insights into regulatory landscapes and procurement processes, they are able to introduce product portfolios tailored to regional preferences and budgetary constraints. Additionally, corporate acquisitions have emerged as a common tactic for integrating complementary capabilities, particularly in the field of respiratory gas monitoring where specialized sensor technologies offer strong differentiation.
Beyond the major incumbent firms, a wave of mid-size and emerging companies is attracting private equity interest through focused portfolios that emphasize digital connectivity and modular design. These innovators are carving out niche positions by delivering specialized consumables, complementary software, and aftermarket support services that extend beyond traditional equipment sales. As investment funds channel growth capital into these ventures, collaboration between established corporations and agile newcomers is expected to intensify, leading to a more dynamic competitive ecosystem.
Innovation pipelines are increasingly focused on sustainability, with companies exploring reusable consumables and modular system architectures that accommodate future upgrades. The development of compact, portable anesthesia units has also gained prominence, reflecting a shared executive belief in the value of flexibility across surgical suites and remote-care environments. Ultimately, these multifaceted strategies underscore the competitive intensity of the market and signal that ongoing collaboration, targeted investment, and agile product development will be critical drivers of success in the years ahead.
Actionable Recommendations for Industry Leaders to Enhance Innovation, Optimize Supply Chains, and Capitalize on Emerging Opportunities in Anesthesia Equipment
Industry leaders in the field of inhalational anesthesia equipment should prioritize the integration of digital health platforms to augment clinical decision support and remote monitoring capabilities. Investing in cloud-based data collection and analytics tools can provide insights into equipment utilization patterns, maintenance needs, and patient outcomes, thereby reinforcing safety protocols and facilitating predictive servicing. In parallel, it is advisable to pursue partnerships with software developers and artificial intelligence specialists to embed machine learning algorithms that enhance dosing accuracy and operational efficiency.Supply chain optimization must also occupy a central role in strategic planning. Establishing regional manufacturing hubs or forging alliances with local contract manufacturers can mitigate the impact of trade tariffs and reduce lead times. Simultaneously, implementing supplier diversification strategies and long-term procurement contracts will help secure access to critical components while protecting against geopolitical disruptions. Scenario planning exercises that model varying tariff regimes can further inform contingency measures and budget forecasting.
Finally, to capitalize on emerging opportunities, organizations should invest in targeted training programs for clinicians and biomedical engineers. By cultivating expertise in new equipment functionalities and maintenance procedures, stakeholders can accelerate adoption rates and reduce equipment downtime. Exploring emerging markets with tailored, cost-effective solutions-such as pneumatic systems for resource-constrained environments-can unlock incremental revenue streams and reinforce market position. Taken together, these actions will ensure that industry leaders remain agile, resilient, and poised for sustainable growth.
Comprehensive Research Methodology Illustrating Data Collection, Validation Techniques, and Analytical Framework for Inhalational Anesthesia Equipment Insights
This analysis draws on a multifaceted research methodology designed to ensure a rigorous and holistic assessment of the inhalational anesthesia equipment landscape. The initial phase involved extensive secondary research, including a review of regulatory filings, white papers, and technical standards documents to establish a foundational understanding of product specifications, compliance requirements, and emerging trends. Concurrently, proprietary industry databases were consulted to map competitive activity and identify technological advancements in machine design, vaporizers, and respiratory gas monitors.The subsequent primary research phase encompassed in-depth interviews with subject matter experts, including anesthesiologists, biomedical engineers, procurement managers, and regulatory specialists. These discussions provided qualitative insights into clinical usage patterns, decision-making criteria, and supply chain challenges. To validate these perspectives, quantitative surveys were deployed across a representative sample of hospital networks and ambulatory centers in key regions, ensuring that findings reflected real-world constraints and preferences.
Data triangulation techniques were employed to reconcile information across sources, with discrepancies addressed through follow-up consultations and cross-referencing against industry benchmarks. The analytical framework incorporated SWOT analysis, competitive position mapping, and supply chain risk assessments to generate actionable insights. Overall, the methodological rigor of this approach underpins the credibility of the conclusions, enabling stakeholders to base strategic decisions on a comprehensive and validated body of evidence.
Concluding Reflections on the Current State and Prospective Evolution of Inhalational Anesthesia Equipment in a Dynamic Healthcare Environment
As surgical disciplines evolve and patient expectations heighten, inhalational anesthesia equipment stands at the nexus of technological innovation, environmental stewardship, and clinical precision. The integration of digital controls, advanced respiratory gas monitoring, and sustainable design principles has redefined the benchmarks for safety and efficiency. Organizations that adopt modular systems capable of remote diagnostics and predictive maintenance will gain a competitive edge by reducing downtime and enhancing patient throughput.At the same time, trade policy shifts such as the United States tariff changes slated for 2025 underscore the necessity of agile supply chain strategies. Companies that proactively localize production or diversify sourcing will be better equipped to navigate cost pressures and potential disruptions. Furthermore, the segmentation of product offerings by system configuration, technology type, end user, and application reveals distinct pathways for targeted growth-ranging from ambulatory surgical centers in developed markets to veterinary applications and emerging economies within Asia-Pacific.
Ultimately, this report illustrates that the future of inhalational anesthesia equipment will be defined by the confluence of clinical demand for safety, regulatory imperatives for environmental impact reduction, and operational pressures to optimize cost structures. Industry leaders who align innovation roadmaps with evolving market needs and geopolitical realities will be positioned to drive sustainable value creation and foster long-term advancement in patient care.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Anesthesia Machines
- Computer-Controlled Anesthesia Machines
- Continuous Flow Anesthesia Machines
- Portable Anesthesia Machines
- Breathing Circuits
- Respiratory Gas Monitors
- Vaporizers
- Anesthesia Machines
- System Type
- Closed Semi Closed Systems
- Open Systems
- Technology
- Electronic
- Mechanical
- Pneumatic
- End User
- Ambulatory Surgical Centers
- Clinics
- Hospitals
- Research Institutes
- Application
- Dental Procedures
- Emergency Medicine
- Pain Management
- Surgery
- Cardiovascular
- Neurosurgery
- Orthopedic
- Veterinary Use
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Aetos Pharma Private Limited
- B. Braun Melsungen AG
- Baxter International, Inc.
- Becton, Dickinson and Company
- Drägerwerk AG & Co. KGaA
- Fisher & Paykel Healthcare Limited
- Fresenius Kabi AG
- GE HealthCare Technologies, Inc.
- Getinge AB
- Halocarbon Life Sciences, LLC
- Hikima Pharmaceuticals plc.
- Löwenstein Medical SE & Co. KG
- Lunan Pharmaceutical Group Co. Ltd
- Mercury Medical LLC
- Midmark Corporation
- Mindray Medical International Limited
- Penlon Limited
- Shenzhen Aeonmed Co., Ltd.
- Shenzhen Mindray Bio‑Medical Electronics Co., Ltd.
- Skanray Technologies, Inc.
- Smiths Medical, Inc.
- Spacelabs Healthcare LLC
- SternMed GmbH
- Teleflex Incorporated
- Vyaire Medical, Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Inhalational Anesthesia Equipment market report include:- Aetos Pharma Private Limited
- B. Braun Melsungen AG
- Baxter International, Inc.
- Becton, Dickinson and Company
- Drägerwerk AG & Co. KGaA
- Fisher & Paykel Healthcare Limited
- Fresenius Kabi AG
- GE HealthCare Technologies, Inc.
- Getinge AB
- Halocarbon Life Sciences, LLC
- Hikima Pharmaceuticals plc.
- Löwenstein Medical SE & Co. KG
- Lunan Pharmaceutical Group Co. Ltd
- Mercury Medical LLC
- Midmark Corporation
- Mindray Medical International Limited
- Penlon Limited
- Shenzhen Aeonmed Co., Ltd.
- Shenzhen Mindray Bio‑Medical Electronics Co., Ltd.
- Skanray Technologies, Inc.
- Smiths Medical, Inc.
- Spacelabs Healthcare LLC
- SternMed GmbH
- Teleflex Incorporated
- Vyaire Medical, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 191 |
| Published | November 2025 |
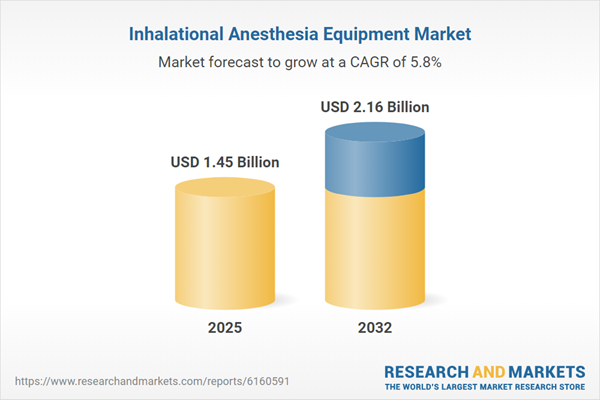
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 1.45 Billion |
| Forecasted Market Value ( USD | $ 2.16 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |