Speak directly to the analyst to clarify any post sales queries you may have.
A comprehensive introduction that frames clinical relevance, engineering evolution, and delivery setting shifts shaping the strategic landscape for hyperbaric oxygen therapy
Hyperbaric oxygen therapy occupies a distinctive niche at the intersection of clinical care, advanced device engineering, and evolving reimbursement and regulatory frameworks. Clinicians increasingly recognize HBOT's role beyond traditional indications, while device manufacturers continue to refine chamber designs, control systems, and patient monitoring to improve safety and usability. At the same time, care delivery is shifting across settings from hospitals toward ambulatory and home environments, creating demand for different pressure ranges and device configurations.Consequently, stakeholders must synthesize clinical evidence, operational requirements, and procurement realities to determine where HBOT can deliver differentiated patient outcomes and economic value. Transitioning from pilot programs to scaled adoption requires collaboration among clinical champions, biomedical engineering teams, and procurement. This report frames those conversations by examining device components, product types, pressure range classifications, clinical applications, end-user environments, and commercial channels to inform decisions on product development, service models, and clinical integration.
Through a rigorous synthesis of industry developments, clinical trends, and supply chain dynamics, this introduction establishes a common baseline for executives, clinical leaders, and strategy teams seeking to translate HBOT potential into operationalized programs that enhance care pathways and patient access.
Transformative clinical, technological, and commercial shifts reshaping delivery models, evidence generation priorities, and the value proposition of hyperbaric oxygen therapy
The landscape for hyperbaric oxygen therapy is undergoing transformative shifts driven by technological advances, changing care delivery models, and heightened attention to clinical evidence and patient experience. Innovations in control systems and gas delivery technologies are improving operational efficiency and enabling safer, more precise treatment protocols. Simultaneously, modular and portable chamber designs have expanded the contexts in which HBOT can be delivered, prompting service providers to rethink traditional site-of-care models.Moreover, healthcare providers are increasingly focused on interdisciplinary care pathways that integrate HBOT with wound care teams, emergency medicine, and specialty clinics, thereby expanding referral networks and optimizing utilization. Regulatory authorities and payers are scrutinizing clinical protocols and outcomes, which elevates the importance of robust, peer-reviewed evidence that demonstrates comparative effectiveness and cost utility. As a result, manufacturers and clinical stakeholders are prioritizing outcomes research and real-world evidence generation to support guideline inclusion and payer discussions.
Finally, commercial dynamics are shifting toward service-oriented propositions, combining device supply with training, maintenance, and telehealth-enabled monitoring. Taken together, these shifts require strategic agility from manufacturers, providers, and distributors to align product roadmaps, clinical research investments, and commercial models with the evolving needs of clinicians and patients.
Potential supply chain reconfiguration and clinical continuity implications arising from cumulative tariff measures and trade policy shifts affecting hyperbaric therapy components
The cumulative effects of tariff actions and trade measures in the United States introduced in and after 2025 have the potential to affect sourcing strategies, component costs, and operational planning for providers and manufacturers involved in hyperbaric oxygen therapy. Imported components such as control systems, specialty valves, and gas management subsystems are central to device performance; changes in tariff policy can therefore alter landed costs and create incentives for supply chain reconfiguration. In response, procurement teams may seek to diversify supplier bases, qualify alternative vendors, and re-evaluate total landed costs rather than relying solely on unit price comparisons.In addition, tariffs can accelerate shifts toward nearshoring or onshoring certain manufacturing activities, particularly for components subject to stringent quality or regulatory requirements. Such localization efforts can yield benefits in lead times and regulatory alignment, yet they may require significant capital investment and operational adjustments. Clinicians and hospital systems may experience indirect effects through procurement cycles and maintenance agreements as suppliers reprice offerings to reflect higher input costs or altered logistics.
Finally, tariff-related policy changes often coincide with broader regulatory and trade conversations that influence availability of spare parts, aftermarket support, and cross-border service provision. Therefore, stakeholders should monitor policy developments closely, engage with trade and regulatory advisors, and implement contingency planning that prioritizes clinical continuity and supply chain resilience while balancing near-term cost pressures with longer-term strategic positioning.
Segmented insights across components, device form factors, pressure classifications, clinical indications, care settings, and commercial channels illuminating adoption levers
Segment-level dynamics provide a granular view of where product innovation, clinical adoption, and commercial strategies intersect across component, product type, pressure range, application, end-user, and sales channel dimensions. Component differentiation matters because Accessories, Control Systems, Gas Supply Systems, and Hyperbaric Chambers carry distinct reliability, service, and regulatory demands that influence total cost of ownership and aftermarket revenue models. Meanwhile, product-type distinctions between Monoplace HBOT devices and Multiplace HBOT devices drive clinical workflow considerations, staffing needs, and capital allocation decisions for facilities pursuing either single-patient throughput or simultaneous multi-patient capacity.Pressure range classifications into High Pressure, Low Pressure, and Medium Pressure categories further determine the clinical use cases and safety protocols appropriate for each device, while clinical applications ranging from Brain Abscesses and Carbon Monoxide Poisoning to Decompression Sickness, Gas Embolism, Infection Treatment, Radiation Tissue Damage, Severe Anemia, and Wound Healing shape referral patterns and protocol standardization. End-user environments such as Ambulatory Surgical Centers, Home Care Settings, Hospitals, and Specialty Centers or Clinics present distinct operational, reimbursement, and patient experience constraints that manufacturers and providers must address through device ergonomics, training offerings, and service packages. Lastly, the interplay between Offline and Online sales channels affects purchase cycles, buyer education, and after-sales engagement, necessitating integrated commercial approaches that align direct sales, distributor partnerships, and digital discovery strategies.
Taken together, these segmentation lenses reveal where product development, clinical evidence generation, and service models can be prioritized to unlock adoption across diverse care settings and use cases.
Regional strategic imperatives that reconcile regulatory diversity, clinical adoption pathways, and supply chain priorities across major global healthcare regions
Regional considerations materially influence clinical adoption pathways, regulatory engagement, and supply chain choices across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, established hospital systems and specialty clinics drive concentrated demand for both monoplace and multiplace chambers, and the regulatory environment encourages structured clinical protocols and reimbursement negotiations that emphasize outcomes and cost-effectiveness. Europe, Middle East & Africa encompasses a wide spectrum of healthcare systems, where pockets of advanced clinical practice coexist with regions prioritizing scalable, lower-cost solutions and partnerships that facilitate technology transfer and training.In the Asia-Pacific region, rapid healthcare infrastructure investment, growing chronic disease prevalence, and increasing private sector participation create opportunities for diversified device configurations and expanded service models. Across all regions, differences in certification pathways, import regulations, and clinical guidelines shape timelines for product introduction and the requirements for local clinical data or adaptations to device features. Consequently, manufacturers and service providers must tailor market entry plans, clinical education efforts, and aftermarket support to regional regulatory expectations and provider capabilities while pursuing partnerships that bridge knowledge gaps and accelerate clinical acceptance.
Regional strategies that incorporate localized training, regulatory alignment, and supply chain redundancy can improve access and ensure continuity of care as devices move from early adopters to broader clinical use.
Competitive and collaborative company-level strategies emphasizing product innovation, service ecosystems, clinical partnerships, and aftermarket differentiation
Competitive dynamics among companies operating in the hyperbaric oxygen therapy ecosystem reflect a balance between product innovation, service delivery excellence, and clinical partnerships. Leading device makers are investing in control system refinements, user interface simplification, and enhanced materials that improve patient comfort and safety. At the same time, companies that complement equipment sales with robust training programs, maintenance contracts, and telehealth-enabled remote support are differentiating on total cost of ownership and clinical uptime.Collaboration between manufacturers and clinical research groups is becoming more prominent as stakeholders recognize the importance of real-world evidence and peer-reviewed studies to broaden indications and secure reimbursement. Strategic partnerships with distribution networks and specialty clinic operators similarly extend market reach while offering local service capabilities that matter for regulated devices. Furthermore, companies that adopt modular product strategies and standardized spare-part ecosystems can streamline regulatory submissions and reduce operational friction for facility biomedical teams.
Finally, new entrants focused on home-care delivery and portable chamber designs are altering competitive dynamics by challenging traditional site-of-care assumptions, prompting incumbents to reassess product portfolios and service models in order to retain clinical and commercial relevance.
Actionable strategic recommendations for device makers, providers, and distributors to accelerate adoption, strengthen resilience, and align commercial models with clinical outcomes
Industry leaders should prioritize a set of actionable initiatives to translate technological progress and clinical promise into sustainable adoption across care settings. First, invest in interoperable control systems and standardized spare-part architectures that simplify maintenance and reduce procurement complexity for facility buyers. Second, accelerate evidence generation that pairs randomized studies with pragmatic real-world data collection to support broader clinical indications and payer engagement.Next, pursue supply chain diversification and regional manufacturing partnerships to mitigate tariff and logistics risks while improving lead times for critical components. Complement these efforts with scalable training programs and telehealth-enabled clinician support to ensure safe adoption in ambulatory and home-care settings. In addition, design commercial models that combine device sales with subscription-style maintenance and outcome-focused service agreements to align incentives among manufacturers, providers, and payers.
Finally, develop targeted go-to-market approaches that reflect segment distinctions-such as distinct offerings for monoplace versus multiplace settings and adaptations for high-, medium-, and low-pressure use cases-and tailor regional strategies to regulatory and reimbursement environments. By implementing these actions, organizations can strengthen clinical impact, operational resilience, and long-term commercial viability.
A multidisciplinary research methodology combining secondary evidence review, stakeholder interviews, and iterative triangulation to produce action-oriented insights and validated conclusions
The research methodology underpinning this analysis combined systematic secondary research with targeted primary engagement and rigorous triangulation to ensure reliability and practical relevance. Secondary research encompassed regulatory documentation, device standards, clinical literature, and publicly available policy updates to establish the technical and clinical context for hyperbaric oxygen therapy. Building on this foundation, primary research included structured interviews with clinicians, biomedical engineers, procurement specialists, and industry executives to surface operational realities, adoption barriers, and service expectations.Data points and qualitative insights were cross-validated through triangulation to reconcile differing perspectives and to highlight consensus areas versus open questions. Segmentation frameworks were applied iteratively, mapping component-level considerations, product-type distinctions, pressure-range implications, application-specific workflows, end-user operational patterns, and sales-channel behaviors. Quality assurance measures included expert review cycles and internal consistency checks to reduce bias and ensure factual accuracy.
Limitations were acknowledged where public evidence is still emerging or where regional regulatory variability constrains broad generalizations. Nevertheless, the methodology prioritized actionable, evidence-informed insights that executives and clinicians can apply to strategic planning, procurement decisions, and clinical program design.
Concluding synthesis highlighting the strategic alignment required between clinical evidence, product design, commercial models, and supply chain resilience to expand HBOT access
In conclusion, hyperbaric oxygen therapy represents a complex convergence of clinical potential, engineering innovation, and evolving care delivery models. The strategic opportunity lies in aligning device development, evidence generation, and commercial models with the practical constraints and incentives of diverse care settings. Clinical adoption will increasingly depend on robust outcomes data, seamless integration into existing care pathways, and service models that lower operational friction for providers.Concurrently, supply chain resilience and regulatory alignment remain critical for ensuring continuity of care as devices and components move across borders and settings. Stakeholders that proactively address these dimensions-through evidence programs, modular product design, diversified sourcing, and targeted regional strategies-will stand in the best position to expand access and improve patient outcomes. Ultimately, the path to broader adoption requires coordinated action across manufacturers, clinical leaders, payers, and distributors to translate technological advances into measurable clinical and economic value.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Component
- Accessories
- Control Systems
- Gas Supply Systems
- Hyperbaric Chambers
- Product Type
- Monoplace HBOT devices
- Multiplace HBOT devices
- Pressure Range
- High Pressure
- Low Pressure
- Medium Pressure
- Application
- Brain Abscesses
- Carbon Monoxide Poisoning
- Decompression Sickness
- Gas Embolism
- Infection Treatment
- Radiation Tissue Damage
- Severe Anemia
- Wound Healing
- End-User
- Ambulatory Surgical Centers
- Home Care Settings
- Hospitals
- Specialty Centers/Clinics
- Sales Channel
- Offline
- Online
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- AHA Hyperbarics GmbH
- CutisCare LLC.
- Environmental Tectonics Corporation
- Gulf Coast Hyperbarics Inc.
- HAUX-LIFE-SUPPORT GmbH
- HEARMEC Corporation Ltd.
- Hyperbaric SAC
- IHC Hytech B.V. (Royal IHC)
- INO Science Inc.
- OxyHealth, LLC
- Perry Baromedical Corp
- SOS Group GBR Ltd
- Sechrist Industries Inc.
- Shanghai Baobang Medical Equipment Co., Ltd.
- Hpotech Hyperbaric Solutions
- HYPERBARIC MODULAR SYSTEMS INC.
- Air Liquide S.A.
- Hytech-Pommec B.V.
- Dremenia GmbH
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Hyperbaric Oxygen Therapy market report include:- AHA Hyperbarics GmbH
- CutisCare LLC.
- Environmental Tectonics Corporation
- Gulf Coast Hyperbarics Inc.
- HAUX-LIFE-SUPPORT GmbH
- HEARMEC Corporation Ltd.
- Hyperbaric SAC
- IHC Hytech B.V. (Royal IHC)
- INO Science Inc.
- OxyHealth, LLC
- Perry Baromedical Corp
- SOS Group GBR Ltd
- Sechrist Industries Inc.
- Shanghai Baobang Medical Equipment Co., Ltd.
- Hpotech Hyperbaric Solutions
- HYPERBARIC MODULAR SYSTEMS INC.
- Air Liquide S.A.
- Hytech-Pommec B.V.
- Dremenia GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
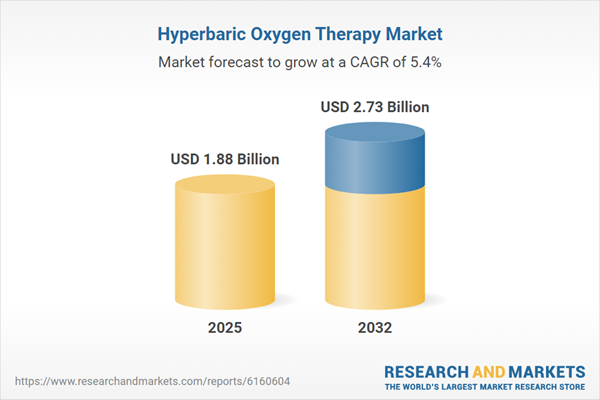
| Estimated Market Value ( USD | $ 1.88 Billion |
| Forecasted Market Value ( USD | $ 2.73 Billion |
| Compound Annual Growth Rate | 5.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |