Speak directly to the analyst to clarify any post sales queries you may have.
Pioneering Innovations and Ethical Imperatives Driving Xenotransplantation from Preclinical Breakthroughs to the Threshold of Clinical Application
Xenotransplantation represents a revolutionary frontier at the intersection of regenerative medicine and transplant surgery, offering the promise to address chronic organ and tissue shortages while reshaping therapeutic paradigms. As patient populations continue to grow and demand for viable transplant options intensifies, the field has witnessed unprecedented scientific breakthroughs in gene editing, immunological tolerance, and bioengineering. These advances have propelled xenotransplantation from theoretical exploration to tangible preclinical success.In recent years, the convergence of CRISPR-driven donor animal modification and novel immunomodulatory regimens has elevated clinical feasibility. Simultaneously, bioethical frameworks and regulatory bodies have evolved to accommodate the unique challenges of cross-species transplantation, prioritizing patient safety and zoonotic risk mitigation. This dynamic landscape underscores the critical need for stakeholders to remain informed of both scientific milestones and emerging policy shifts.
This executive summary distills the most salient developments shaping the xenotransplantation ecosystem. It synthesizes transformative technological innovations, tariff-driven supply chain realignments, granular segmentation insights, regional variations, and key industry players. By articulating these foundational elements, the introduction aims to equip experts and decision-makers with a clear, authoritative vantage point on where the field stands today-and where it is poised to advance tomorrow.
Rewriting the Xenotransplantation Playbook through Gene Editing Advancements and Immunomodulatory Strategies Shaping the Next Generation of Therapeutic Applications
Over the past decade, xenotransplantation has undergone a profound metamorphosis driven by precision gene editing and advanced immunomodulatory techniques. The advent of CRISPR/Cas9 platforms has enabled targeted inactivation of porcine endogenous retroviruses, alongside modifications to reduce immunogenicity. Concurrently, novel monoclonal antibodies and cellular therapies are redefining graft acceptance by modulating recipient immune responses with unprecedented specificity.These scientific strides have been bolstered by innovations in biomanufacturing and organ preservation. Emerging perfusion systems now extend viability windows, while microfluidic modeling of xenografts accelerates translational research. In parallel, three-dimensional bioprinting of scaffolded tissues is charting new pathways for hybrid constructs that integrate decellularized xenogeneic matrices with human cells.
Regulatory frameworks have kept pace with these technological leaps. Adaptive approval pathways and harmonized safety guidelines are facilitating cross-border clinical trials and public-private consortia. As a result, academic centers and biotechnology ventures are collaboratively advancing toward first-in-human studies. These transformative shifts in the xenotransplantation landscape are redefining therapeutic possibilities and heralding a new era of interspecies transplantation science.
Unraveling the Cross-Border Dynamics of US Tariffs in 2025 Impacting Supply Chains Regulatory Collaboration and Cost Structures in Xenotransplantation
In 2025, newly enacted United States tariffs on biotechnology imports and laboratory reagents have created complex implications for the xenotransplantation sector. These measures have directly increased the cost of critical gene editing enzymes, custom animal models, and specialized preservation solutions, prompting many research institutions to reassess supplier portfolios. Consequently, a growing number of laboratories are exploring domestic manufacturing partnerships to mitigate exposure to escalating tariffs.Beyond direct cost pressures, the policy shift has altered collaboration dynamics between US-based developers and international centers of excellence. Joint ventures in Europe and Asia Pacific have been recalibrated to balance intellectual property protections with tariff avoidance strategies. In some instances, preclinical testing is migrating to regions with more permissive trade agreements, while US entities negotiate tariff exemptions tied to innovation incentives.
These evolving trade conditions underscore the importance of strategic supply chain resilience. Organizations are adopting dual-sourcing frameworks for donor animal procurement, diversifying reagent pipelines, and leveraging localized contract research organizations to uphold research continuity. As tariff policies continue to shape cross-border exchanges, stakeholders must proactively adjust operational models to safeguard both innovation momentum and cost-effectiveness within the xenotransplantation domain.
Decoding the Multidimensional Segmentation Landscape Revealing Strategic Insights across Transplant Types Species Sources Clinical Applications and End Users
Insights into transplant types reveal that cell-based approaches are rapidly advancing, with corneal cell therapies demonstrating early clinical promise, hepatocytes enabling targeted liver support, islet cells revolutionizing diabetes management, and neuronal cells opening avenues for neurodegenerative disease interventions. Solid organ xenotransplantation presents a spectrum of complexity; heart and kidney grafts have reached pivotal proof-of-concept milestones, while liver, lung, and pancreas transplants are driving intensive immunomodulation research to overcome rejection barriers. Tissue-based strategies, leveraging decellularized matrices and scaffold technologies, are increasingly integrated into regenerative medicine applications.Examining source species underscores the predominance of porcine donors due to organ size compatibility and genetic malleability. Baboons and chimpanzees remain critical for immunological modeling, although ethical considerations limit their use. Emerging alternatives, such as goats and rabbits, are gaining attention for specific tissue applications, while bovine models support specialized bioengineering efforts.
Application-led segmentation highlights diverse therapeutic arenas. Burn and wound care protocols incorporate xenogeneic skin grafts for accelerated healing. Diabetes management benefits from islet cell encapsulation techniques. Neurodegenerative treatments leverage neuronal xenografts to restore neural networks. Oncology research utilizes xenograft models for tumor biology insights. Organ failure treatments are progressively transitioning from preclinical studies toward clinical trial readiness.
End users shape adoption pathways, with ambulatory surgical centers piloting minimally invasive cell-based procedures. Hospitals and transplant centers drive major organ implant operations, while research institutes and academic medical centers pioneer investigative protocols. Specialty clinics are carving out niche therapies, supported by consultative transplant coordination.
Navigating Diverse Regional Ecosystems Highlighting Innovation Drivers and Regulatory Frameworks in the Americas EMEA and Asia-Pacific Territories
The Americas region continues to lead in xenotransplantation research, propelled by robust academic-industry partnerships and favorable regulatory frameworks. The United States and Canada spearhead first-in-human trial preparations, leveraging a mature biotech ecosystem and substantial research funding. Latin American centers are progressively engaging through collaborative grant mechanisms and building capacity for preclinical studies, fostering a broad innovation network across the hemisphere.In Europe, regulatory harmonization under the European Medicines Agency has accelerated pathway clarity, enabling multinational trial consortia. Ethical oversight committees have established rigorous zoonotic risk protocols, setting global benchmarks. The Middle East, buoyed by significant healthcare investments, is developing state-of-the-art breeding facilities for genetically engineered donors, while African institutions are prioritizing biosecurity measures and foundational research to address transplantation gaps.
Asia-Pacific markets are witnessing exponential growth, led by China’s ambitious gene editing initiatives and Japan’s advanced biomanufacturing capabilities. Australia is forging stringent safety guidelines, positioning itself as a secure testing ground. India and Southeast Asia are balancing resource constraints with a high prevalence of organ failure, adopting cost-effective xenograft models and forming international collaborations to accelerate translational research across the region.
Profiling Leading Innovators and Strategic Partnerships Forging the Future of Xenotransplantation with Cutting-Edge Research Pipelines and Alliance Models
Leading biotechnology and medical research enterprises are driving the xenotransplantation agenda through integrated R&D pipelines and strategic alliance models. Several firms with expertise in immunology have formed partnerships with organ preservation specialists to co-develop next-generation perfusion systems. At the same time, cell therapy companies are collaborating with contract breeding organizations to secure pathogens-free donor herds tailored to specific immunogenetic profiles.Strategic investments by venture capital and pharmaceutical investors have fostered joint ventures, enabling access to specialized bioprocessing infrastructure and accelerating candidate selection for first-in-human studies. Industry leaders are also building precompetitive consortiums to standardize safety assessments, pooling data on cross-species viral transmission and immunogenicity biomarkers. This collective approach reduces redundancy and facilitates regulatory engagement.
Recent mergers and acquisitions have consolidated expertise across gene editing, immunomodulation, and biofabrication domains. These transactions underscore a trend toward vertically integrated models that span donor animal development, therapeutic design, and clinical trial execution. As these companies refine their portfolios, they are increasingly focused on scalable manufacturing, quality assurance frameworks, and global market entry strategies to translate scientific breakthroughs into viable clinical solutions.
Implementing Targeted Strategies for Industry Leaders to Navigate Regulatory Complexities Drive Innovation and Cultivate Sustainable Growth in Xenotransplantation
Industry leaders should prioritize the establishment of multidisciplinary innovation ecosystems, bringing together geneticists, immunologists, bioengineers, and regulatory experts to drive seamless translational progress. By integrating early-stage gene editing programs with advanced immunomodulatory research, organizations can shorten development cycles and bolster clinical readiness. Equally important is the cultivation of public-private partnerships to distribute risk and share intellectual property in precompetitive domains.To navigate evolving regulatory complexities, executives must engage proactively with health authorities, contributing to the refinement of safety standards and ethical guidelines. Establishing dedicated regulatory affairs teams with specialized xenotransplantation expertise will ensure timely submissions, adaptive trial designs, and global harmonization. Robust risk management frameworks, incorporating zoonotic surveillance and contingency plans, will reinforce stakeholder confidence and protect patient welfare.
Sustainable growth depends on creating resilient supply chains for genetically tailored donor herds and critical reagents. Diversifying sourcing strategies, investing in localized manufacturing capacities, and pursuing tariff mitigation agreements will defend against geopolitical disruptions. Finally, transparent stakeholder communication and community engagement initiatives are essential to build public trust and ethical stewardship, underpinning long-term acceptance and market adoption.
Establishing Rigorous Primary and Secondary Research Methods to Ensure Data Integrity Expert Validation and Adaptable Analytical Frameworks
Our research methodology combines rigorous primary and secondary approaches to ensure comprehensive, validated insights. Primary research elements include in-depth interviews with transplant surgeons, immunologists, biotech executives, and regulatory authorities. These discussions provide qualitative context around technological feasibility, clinical adoption challenges, and policy evolution. Site visits to preclinical laboratories and breeding facilities supplement firsthand observations of operational workflows and quality controls.Secondary research encompasses a structured review of peer-reviewed journals, patent filings, regulatory filings, and public company disclosures. Data triangulation techniques reconcile divergent sources, strengthening the reliability of thematic findings. Quantitative inputs derived from scientific publications and industry reports are subjected to cross-verification against expert feedback to mitigate information biases and validate assumptions.
The analytic framework follows a structured process, encompassing segmentation analysis, regional mapping, tariff impact assessment, and competitive landscape profiling. Iterative validation sessions with key opinion leaders and external consultants refine the conclusions. By combining methodological rigor with expert interpretation, the study delivers a robust foundation for strategic decision-making in xenotransplantation.
Synthesizing Key Findings to Illuminate Pathways for Technological Advancement Ethical Oversight and Stakeholder Collaboration in Xenotransplantation
This executive summary synthesizes the most critical developments shaping the xenotransplantation field, from gene editing innovations to supply chain realignments driven by new tariff policies. By examining detailed segmentation insights, we have illuminated key opportunities across cell-based, solid organ, and tissue-based modalities, as well as diversified species sources and end-user adoption pathways.Regional analyses reveal distinct dynamics: the Americas lead in clinical trial readiness, Europe and the Middle East are refining regulatory and ethical frameworks, and Asia-Pacific markets are rapidly expanding through ambitious R&D investments. Competitive profiling highlights collaborative models and integrated pipelines that are essential for accelerating translational progress.
Moving forward, stakeholders must adopt agile strategies that address regulatory uncertainties, fortify supply chains, and foster open innovation ecosystems. Embracing robust research methodologies and engaging transparently with stakeholders will be paramount to realizing the full therapeutic potential of xenotransplantation. Collectively, these aligned efforts will advance this transformative domain from experimental promise to mainstream clinical practice.
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Transplant Type
- Cell-based Xenotransplantation
- Corneal Cells
- Hepatocytes
- Islet Cells
- Neuronal Cells
- Solid Organ Xenotransplantation
- Heart
- Kidney
- Liver
- Lungs
- Pancreas
- Tissue-based Xenotransplantation
- Cell-based Xenotransplantation
- Source Animal Species
- Baboons
- Chimpanzees
- Cows
- Goats
- Pig
- Rabbits
- Application
- Burn & Wound Care
- Diabetes Management
- Neurodegenerative Diseases
- Oncology
- Organ Failure Treatment
- End User
- Ambulatory Surgical Centers
- Hospitals
- Research Institutes & Academic Medical Centers
- Specialty Clinics
- Transplant Centers
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Astellas Pharma, Inc
- eGenesis, Inc.
- F. Hoffmann-La Roche Ltd.
- Immerge BioTherapeutics, Inc.
- Infigen, Inc.
- Makana Therapeutics
- Novartis AG
- NZeno Limited
- OrganOX Limited
- Pfizer, Inc
- Preservation Solutions, Inc.
- Qihan Biotech
- Revivicor, Inc.
- Sernova Corp.
- Xenothera SA
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Xenotransplantation market report include:- Astellas Pharma, Inc
- eGenesis, Inc.
- F. Hoffmann-La Roche Ltd.
- Immerge BioTherapeutics, Inc.
- Infigen, Inc.
- Makana Therapeutics
- Novartis AG
- NZeno Limited
- OrganOX Limited
- Pfizer, Inc
- Preservation Solutions, Inc.
- Qihan Biotech
- Revivicor, Inc.
- Sernova Corp.
- Xenothera SA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 195 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
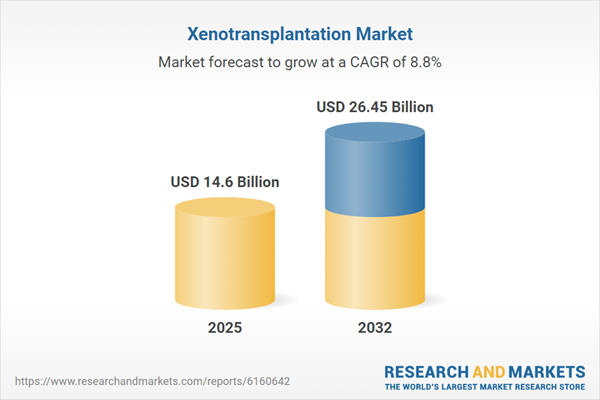
| Estimated Market Value ( USD | $ 14.6 Billion |
| Forecasted Market Value ( USD | $ 26.45 Billion |
| Compound Annual Growth Rate | 8.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |