Speak directly to the analyst to clarify any post sales queries you may have.
Overview of the Global Pressure Monitoring Market Providing Context for Key Trends Technological Advances and Clinical Drivers Shaping Industry Growth
Pressure monitoring technologies play a pivotal role across diagnostic and therapeutic settings by providing the measurements clinicians rely on to guide critical decisions. The measurement of blood, intracranial, intraocular, and pulmonary pressures has evolved significantly as continuous monitoring gains prominence over sporadic checks. In modern healthcare environments, real-time data streams empower medical professionals to detect deviations early and adjust treatment protocols dynamically, fostering improved outcomes and patient safety.Furthermore, rising chronic disease prevalence and the demands of an aging population have shifted the focus from acute inpatient care to long-term management strategies. As traditional hospital settings make room for ambulatory surgical centers, outpatient clinics, diagnostic laboratories, and home care environments, pressure monitoring systems must adapt in form factor and functionality. This geographical and care-setting decentralization underscores the importance of flexible solutions that accommodate varying clinical workflows and patient mobility requirements.
Recent technological strides in sensor miniaturization, materials engineering, and wireless connectivity are redefining the capabilities of both accessories and devices. Integration with electronic health records and cloud-based analytic platforms has unlocked new pathways for data sharing, trend analysis, and predictive modeling. As a result, the pressure monitoring ecosystem stands at the nexus of medical innovation and data-driven care delivery, poised for continued transformation.
Emerging Technological Innovations Market Consolidation and Evolving Clinical Practices Redefining the Future Landscape of Pressure Monitoring Solutions
Emerging advances in pressure monitoring reflect a confluence of technological innovation, corporate consolidation, and evolving clinical protocols. Connected devices that leverage the Internet of Medical Things now offer continuous remote surveillance, while wearable patches and wireless catheter sensors facilitate patient mobility without compromising data integrity. Miniaturized electronics and low-power components have enabled the design of discreet devices that seamlessly integrate into care regimens, enhancing both user comfort and measurement accuracy.In parallel, artificial intelligence and machine learning algorithms are being embedded within analytic platforms to transform raw pressure data into actionable insights. Predictive modeling tools can identify patterns that precede adverse events, equipping care teams with early warnings that mitigate risk. As integration with electronic health records deepens, clinicians can access longitudinal pressure trends side by side with other vital parameters, promoting a holistic view of patient health.
This wave of digital transformation has accelerated strategic partnerships, mergers, and acquisitions among device manufacturers, technology providers, and healthcare systems. Collaborative R&D initiatives now span disciplines, from biomedical engineering to data science, unlocking synergistic potential that drives next-generation product roadmaps. As alliances continue to reshape competitive boundaries, end users can expect more comprehensive and interoperable solutions.
Moreover, value-based care models and remote patient management programs are redefining clinical practice. Increasingly, providers are adopting telehealth strategies that rely on accurate pressure monitoring outside hospital walls. This clinical shift toward decentralized, patient-centric models is a testament to the transformative impact of the convergence between medical technology and digital health services.
Evaluation of United States Tariff Adjustments of 2025 and Their Wide-Ranging Consequences for Manufacturing Expenditures Supply Networks and Clinical Access
The imposition of new United States tariffs in 2025 has introduced a complex layer of cost considerations for manufacturers and end users in the pressure monitoring ecosystem. With components sourced from global suppliers now subject to higher import duties, original equipment manufacturers face rising production expenses that ripple across the supply chain. In response, many suppliers are revisiting procurement strategies, exploring alternative sourcing regions, and negotiating long-term agreements to stabilize material costs.However, the immediate aftermath of tariff implementation has been characterized by logistical bottlenecks and extended lead times for critical components. Tier-one suppliers have adjusted inventory buffers to mitigate disruptions, yet downstream distributors and healthcare providers have encountered sporadic availability challenges. As a result, certain stakeholders are accelerating qualification processes for domestic suppliers or investing in localized contract manufacturing arrangements to maintain continuity of device production and delivery.
From a distribution perspective, channel partners are recalibrating stocking strategies to absorb tariffs without eroding margins. Online and offline outlets alike are reassessing pricing structures, often sharing incremental cost burdens across wholesale, retail, and direct-sale models. Consequently, transparent communication and collaborative inventory planning have emerged as essential tactics for minimizing clinical service interruptions.
Looking forward, organizations are exploring design optimizations to reduce reliance on tariff-exposed components. By embracing modular architectures and vendor-agnostic interfaces, developers can reengineer product lines with greater agility. In tandem, strategic partnerships aimed at nurturing supply-chain resilience and regulatory alignment will be critical for navigating the long-term implications of the 2025 tariff landscape.
In-Depth Examination of Market Segmentation Reveals Insights Across Product Types Portability Procedures Interfaces End-Use Channels and Clinical Applications
A nuanced understanding of market segmentation reveals distinct drivers and preferences that shape product development and deployment strategies. The pressure monitoring industry is structured around core product categories, with accessories encompassing calibration kits, cuffs, disposables, and tubing, while devices include blood pressure monitors, intracranial pressure modules, intraocular gauges, and pulmonary pressure systems. This bifurcation underscores the importance of harmonizing component reliability with device precision to meet varied clinical requirements.Portability represents another critical segmentation dimension. Portable and handheld pressure monitoring instruments support point-of-care applications and home-based supervision, whereas stationary platforms are often entrenched in intensive care units and surgical suites. Balancing mobility and robustness in device architecture ensures that manufacturers cater to healthcare settings ranging from ambulatory centers to high-acuity hospitals.
Procedure-based distinctions further differentiate market opportunities, with invasive monitoring solutions deeply integrated into surgical and critical care protocols, contrasted by non-invasive options favored in outpatient diagnostics and routine health assessments. Interface preferences also factor prominently, as analog readouts remain prevalent in resource-constrained environments, even as digital displays and connectivity features gain traction in advanced facilities.
End-use segmentation highlights the varying volumes and service models across ambulatory surgical centers and clinics, diagnostic laboratories, home care programs, and hospital infrastructures. Distribution channels similarly span offline networks-comprising direct sales teams and distributor partnerships-and online touchpoints, including company portals and eCommerce platforms. Finally, application-driven demand arises from cardiac monitoring, dialysis support, glaucoma management, neurological diagnostics, and respiratory care, each category reinforcing the necessity for specialized pressure monitoring capabilities.
Comparative Regional Analysis Highlighting Distinct Drivers Barriers and Growth Opportunities in the Americas Europe Middle East Africa and Asia-Pacific Markets
Regional dynamics exert a profound influence on the adoption and evolution of pressure monitoring technologies. In the Americas, advanced healthcare systems in the United States and Canada benefit from established reimbursement frameworks and substantial investments in medical device infrastructure. High demand for remote patient monitoring and home-based diagnostics has catalyzed collaborations between device makers and telehealth providers, while Latin American markets are witnessing gradual uptake driven by government health initiatives and expanding private-sector partnerships.Within Europe, Middle East & Africa, a mosaic of regulatory landscapes and healthcare funding models shapes regional trends. Western European countries prioritize innovative digital solutions and value-based procurement, supported by stringent device approval pathways. Concurrent challenges in parts of the Middle East and Africa, including infrastructure bottlenecks and limited specialist access, have prompted targeted pilot programs that emphasize portable monitoring and simplified user interfaces.
Asia-Pacific markets exhibit diverse trajectories, with high-income economies like Japan and Australia leading adoption of next-generation pressure monitoring systems, integrating predictive analytics and cloud connectivity. In contrast, emerging economies across Southeast Asia and India are accelerating local manufacturing and distribution to align with cost-sensitive healthcare models. Government subsidies and public-private partnerships are driving expanded access, particularly in home care and community health settings.
Across all regions, stakeholders are prioritizing interoperability, data security, and regulatory compliance as foundational elements for scaling pressure monitoring solutions. Cross-border knowledge exchanges and standardization efforts continue to shape the competitive landscape and inform strategic investments.
Insightful Profile of Top Pressure Monitoring Companies Highlighting Their Product Diversification Strategic Alliances and Technology Innovation Trajectories
Leading participants in the pressure monitoring sector are pursuing multifaceted strategies to differentiate their offerings and maintain competitive advantage. Several established medical device manufacturers have diversified their product portfolios to include both traditional measurement modules and advanced digital platforms that integrate seamlessly with electronic health records. This dual approach ensures relevance across a spectrum of care settings, from bedside monitoring to remote patient surveillance.Innovation pipelines have become a focal point for differentiation, with companies investing heavily in next-generation sensor technologies, wireless connectivity, and embedded analytics. Collaborative alliances with technology firms, academic institutions, and healthcare systems facilitate rapid prototyping and validation of novel solutions. In parallel, strategic acquisitions are enabling certain players to augment their capabilities in data management and cloud infrastructure, thereby creating end-to-end monitoring ecosystems.
Partnership models have also extended to aftermarket services and support, as organizations recognize that holistic customer engagement-including training programs, software updates, and maintenance agreements-can drive long-term loyalty. By fostering close relationships with clinical teams and procurement professionals, device manufacturers are able to tailor solutions that address workflow inefficiencies and regulatory requirements more effectively.
Furthermore, a growing number of industry leaders are exploring opportunities in value-based care initiatives, aligning their business models with outcomes-driven reimbursement frameworks. This shift underscores a broader market transformation, in which pressure monitoring solutions are positioned not only as diagnostic tools but as integral components of population health management strategies.
Strategic Imperatives for Leaders to Capitalize on Emerging Pressure Monitoring Innovations Enhance Supply Chain Resilience and Accelerate Clinical Adoption
To capitalize on emerging opportunities and navigate evolving market dynamics, industry leaders should prioritize the enhancement of digital capabilities and interoperability frameworks. Integrating pressure monitoring devices with electronic health records and telehealth platforms will facilitate seamless data exchange and support remote patient management initiatives. In tandem, investing in modular product designs that accommodate rapid component upgrades can accelerate time-to-market for next-generation sensors and analytic software.Strengthening supply chain resilience is equally critical. Organizations should cultivate diversified supplier networks, establish regional manufacturing partnerships, and implement advanced inventory forecasting tools. These measures will mitigate the impact of future external shocks, such as tariff modifications or logistical disruptions, while preserving cost efficiency.
Clinical adoption can be further advanced through targeted training programs and evidence-based protocols that underscore the value of continuous pressure monitoring in diagnostic and therapeutic pathways. Engaging key opinion leaders and payers in pilot initiatives can help validate outcomes, inform reimbursement strategies, and reinforce the clinical efficacy of innovative monitoring solutions.
Finally, forging strategic alliances with telemedicine providers, software developers, and regulatory consultants will enable device manufacturers to respond rapidly to shifting end-user requirements. By fostering a collaborative ecosystem that spans research institutions and healthcare delivery networks, companies can position themselves at the forefront of pressure monitoring innovation and deliver tangible value across the continuum of care.
Comprehensive Research Methodology Employing Primary Interviews Secondary Data Synthesis and Validation to Produce Robust Insights on Pressure Monitoring Trends
This report is underpinned by a rigorous research methodology designed to ensure both depth and accuracy of insights. Primary interviews were conducted with a diverse array of stakeholders, encompassing clinicians, procurement managers, biomedical engineers, and regulatory experts. These qualitative engagements provided nuanced perspectives on device performance, clinical workflows, and decision-making criteria.Secondary research efforts included a comprehensive review of publicly available literature, including regulatory filings, clinical trial registries, industry publications, and peer-reviewed journals. This synthesis of secondary data produced a robust contextual framework that complements the firsthand insights gathered through primary investigations.
Data validation procedures involved cross-referencing interview findings with documented case studies and benchmark reports. Discrepancies were methodically addressed through follow-up consultations, ensuring that all conclusions reflect a high degree of consensus among industry participants. Throughout the process, adherence to ethical research standards and quality-assurance protocols was paramount.
The combination of systematic data collection, meticulous verification, and multistakeholder analysis yields a comprehensive view of the pressure monitoring market. This methodology empowers decision-makers with a balanced understanding of technological trends, regulatory influences, and competitive dynamics, supporting strategic planning and investment decisions.
Final Reflections on the Evolving Pressure Monitoring Landscape Highlighting Core Takeaways Strategic Priorities and the Road Ahead for Industry Stakeholders
The pressure monitoring landscape is characterized by rapid technological advancements, shifting clinical paradigms, and evolving regulatory environments. Key trends, including the integration of digital connectivity, sensor miniaturization, and data analytics, are redefining how healthcare providers measure and respond to physiological pressures. Moreover, the decentralization of care-from hospitals to home settings-continues to expand the use cases for both devices and accessories.External factors, such as the 2025 tariff adjustments in the United States, have highlighted the importance of resilient supply chains and strategic sourcing. In response, stakeholders are redesigning procurement practices and pursuing localized manufacturing to maintain operational continuity. Simultaneously, segmentation analysis underscores the necessity for tailored solutions across product types, portability formats, procedural applications, interfaces, end-use environments, distribution channels, and clinical areas ranging from cardiac care to neurological diagnostics.
Regional markets display distinct adoption patterns influenced by reimbursement frameworks, regulatory pathways, infrastructure maturity, and healthcare priorities. North America, Europe, and parts of Asia-Pacific lead with advanced digital implementations, while emerging economies focus on cost-effective and portable systems. Competitive dynamics are shaped by a combination of established medical device manufacturers, technology entrants, and strategic alliances, all striving to differentiate through innovation pipelines and value-added services.
To thrive in this dynamic environment, companies must embrace interoperable technologies, cultivate strategic partnerships, and align product development with clinical needs. By applying the actionable recommendations outlined in this report, industry participants can position themselves for sustained success and drive the next wave of innovation in pressure monitoring.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Accessories
- Calibration Kits
- Cuffs
- Disposables
- Tubing
- Devices
- Blood Pressure Monitors
- Intracranial Pressure Monitors
- Intraocular Pressure Monitors
- Pulmonary Pressure Monitors
- Accessories
- Device Portability
- Portable/Handheld Devices
- Stationary
- Procedure
- Invasive Monitoring
- Non-invasive Monitoring
- Interface
- Analog
- Digital
- End Use
- Ambulatory Surgical Centers and Clinics
- Diagnostic Laboratories
- Home Care Settings
- Hospitals
- Distribution Channel
- Offline
- Direct Sale
- Distributor Network
- Online
- Company Websites
- eCommerce Platforms
- Offline
- Applications
- Cardiac Disorders
- Dialysis
- Glaucoma
- Neurological Disorders
- Respiratory Disorders
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- A&D Medical Inc.
- ABBOTT INC.
- American Diagnostics Corp.
- Baxter International
- BECTON, DICKINSON AND COMPANY
- BOSTON SCIENTIFIC CORPORATION
- Briggs Healthcare
- Carl Zeiss AG
- DRÄGERWERK AG & CO. KGAA
- GE Healthcare
- HONSUN
- Integra LifeSciences Holdings Corporation
- Koninklijke Philips N.V.
- Medtronic PLC
- Nihon Kohden Corporation
- Omron Healthcare Welch Allyn, Inc.
- Rossmax International Ltd.
- Spacelabs Healthcare Inc.
- SunTech Medical, Inc.
- TERUMO CORPORATION
- Withings
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Pressure Monitoring market report include:- A&D Medical Inc.
- ABBOTT INC.
- American Diagnostics Corp.
- Baxter International
- BECTON, DICKINSON AND COMPANY
- BOSTON SCIENTIFIC CORPORATION
- Briggs Healthcare
- Carl Zeiss AG
- DRÄGERWERK AG & CO. KGAA
- GE Healthcare
- HONSUN
- Integra LifeSciences Holdings Corporation
- Koninklijke Philips N.V.
- Medtronic PLC
- Nihon Kohden Corporation
- Omron Healthcare Welch Allyn, Inc.
- Rossmax International Ltd.
- Spacelabs Healthcare Inc.
- SunTech Medical, Inc.
- TERUMO CORPORATION
- Withings
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
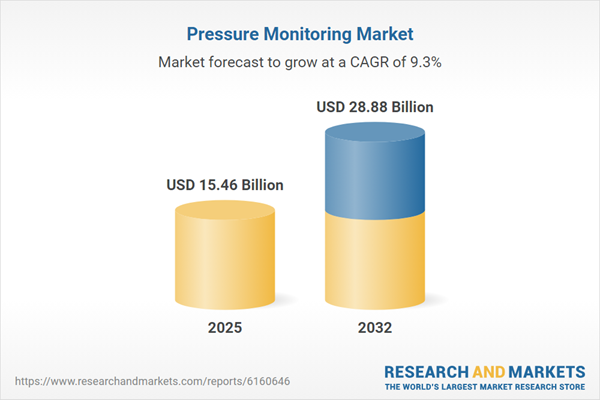
| Estimated Market Value ( USD | $ 15.46 Billion |
| Forecasted Market Value ( USD | $ 28.88 Billion |
| Compound Annual Growth Rate | 9.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 22 |