Speak directly to the analyst to clarify any post sales queries you may have.
Comprehensive Overview of Technological Advancements and Clinical Importance of Titanium Micro Forceps Revolutionizing Precision in Minimally Invasive Surgeries
The evolution of surgical instruments has never been more dynamic, and titanium micro forceps stand at the forefront of this transformation. In recent years, the industry has witnessed a decisive shift toward materials that offer exceptional strength-to-weight ratios, superior biocompatibility, and long-term durability. Titanium, known for its corrosion resistance and nonmagnetic properties, has become the material of choice for micro forceps used in microscale surgical procedures. As minimally invasive techniques continue to gain traction, surgeons rely increasingly on precision instruments that can deliver reliable performance in the most delicate settings.Emerging from a heritage of stainless steel tools, titanium micro forceps are redefining the standards for microsurgical interventions across multiple medical disciplines. Their ergonomic designs minimize hand fatigue during prolonged procedures, while advanced manufacturing processes ensure consistent tip tolerances down to the submillimeter level. Moreover, innovations in surface treatments have enhanced sterilization efficacy, reducing the risk of post-operative complications. Consequently, hospitals, specialty clinics, and ambulatory surgery centers are integrating these instruments into their surgical suites to meet rising patient expectations for safety and precision.
Looking ahead, the market landscape will be shaped by converging trends in customization, sustainability, and digital integration. As healthcare providers strive for cost-effective yet high-performance solutions, the momentum behind titanium micro forceps is set to accelerate. In this context, a comprehensive understanding of technological, regulatory, and commercial drivers is essential for stakeholders aiming to navigate this rapidly evolving domain.
Analyzing the Major Technological Innovations and Regulatory Developments Reshaping the Titanium Micro Forceps Market Landscape
Innovation in the titanium micro forceps market has accelerated over the past decade, driven by breakthroughs in manufacturing techniques and regulatory evolution. The adoption of additive manufacturing has enabled the production of intricate geometries that were previously unachievable with conventional machining. This has opened the door to ergonomic handle designs and ultra-fine tip configurations that cater to the exacting demands of microsurgical applications. Complementing these advancements, novel surface coatings have enhanced instrument longevity by providing superior resistance to wear and corrosion.Simultaneously, regulatory frameworks in key markets have undergone significant updates that directly influence product development cycles and market entry strategies. Guidance from leading health authorities now mandates more rigorous biocompatibility testing and post-market surveillance requirements, compelling manufacturers to refine their quality management systems and documentation processes. In parallel, an increasing emphasis on sustainability has prompted a reevaluation of single-use versus reusable forceps, with a growing number of healthcare institutions implementing circular economy principles to reduce medical waste without compromising on sterility and performance.
As a result of these combined forces, the competitive landscape is becoming more dynamic. Established medical device firms are forming strategic alliances with specialized technology providers to co-create next-generation instruments, while startups are securing venture funding to bring disruptive concepts to market. Against this backdrop, stakeholders must stay attuned to regulatory signals, invest in advanced production capabilities, and forge collaborations that align technological potential with clinical needs.
Examining the Consolidated Effects of New US Tariffs on Titanium Micro Forceps Supply Chains and Manufacturing Dynamics in 2025
In early 2025, the implementation of new tariffs on imported surgical instruments by the United States government introduced a complex array of challenges and opportunities for manufacturers and distributors of titanium micro forceps. The revised duty schedules have increased the landed cost for instruments sourced from traditional offshore hubs, prompting procurement teams to reexamine their supply chain footprints. Hospitals and specialty clinics have begun to experience elevated purchasing expenses, driving demand for localized production strategies and alternative sourcing arrangements.Manufacturers are responding by diversifying their supplier base across the Americas and Asia Pacific, while some are accelerating plans to establish regional assembly operations to mitigate tariff impacts. This strategic shift not only alleviates immediate cost pressures but also enhances agility in responding to fluctuating demand patterns. Meanwhile, distributors are negotiating tariff classifications and duty deferral mechanisms, seeking to maintain pricing stability for their healthcare customers.
Despite short-term cost inflation, the long-term outlook suggests that supply chain realignment could yield efficiency gains through nearshoring and lean inventory practices. Furthermore, collaborative initiatives between device makers and healthcare providers are emerging to co-develop instruments that balance performance requirements with cost containment. As stakeholders navigate this evolving tariff environment, proactive supply chain optimization and alternative manufacturing models will be essential to sustaining competitiveness.
Unveiling Critical Market Segmentation Insights by Product Type, Application Area, End User Profile, and Sales Channel for Titanium Micro Forceps
A nuanced view of the titanium micro forceps market emerges when dissecting it along multiple axes of segmentation. By product type, demand surges for angled and curved micro forceps have been fueled by their enhanced maneuverability in confined surgical fields, while straight forceps remain a staple in many operating rooms due to their versatility. Customized micro forceps, available in both one-time-use and reusable variants, are gaining traction among institutions that prioritize tailored instrument configurations and streamlined sterilization protocols.From the application perspective, the dental surgery segment encompasses endodontic procedures demanding fine-tipped forceps, implantology applications requiring robust grip mechanisms, and periodontal interventions that challenge instrument precision. In neurosurgery, spinal surgeries depend on specialized micro forceps for delicate tissue manipulation, whereas tumor resection and vascular repair procedures demand instruments calibrated to minimize trauma. Ophthalmic surgery drives significant uptake, with cataract, corneal, glaucoma, and retinal operations each influencing specific design attributes. Plastic surgery applications, whether cosmetic enhancements or reconstructive techniques, further diversify functional requirements.
Turning to end users, the market spans ambulatory surgery centers that seek cost-effective disposable options, hospitals that invest in high-performance reusable sets, and specialty clinics-ranging from dental facilities to ophthalmic practices and private physician offices-that demand application-specific instrument portfolios. Sales channel dynamics underscore the importance of direct sales relationships for high-value accounts, while distributors-both local and national-extend reach into smaller facilities, and online retailers offer convenient access for emerging clinics and research institutions.
Highlighting Distinct Regional Dynamics and Growth Drivers in the Americas, Europe Middle East Africa, and Asia Pacific Titanium Micro Forceps Markets
The Americas region, led by the United States and Canada, continues to dominate demand for titanium micro forceps owing to its advanced healthcare infrastructure, widespread adoption of minimally invasive procedures, and emphasis on surgical precision. Investment in local manufacturing capabilities has accelerated as stakeholders seek to reduce import dependency and respond swiftly to clinician preferences. Meanwhile, healthcare providers throughout Latin America are gradually increasing their use of specialized instruments, driven by improvements in procedural training and expanding insurance coverage.In Europe, Middle East, and Africa, the regulatory convergence within the European Union has elevated quality standards for medical devices, prompting suppliers to secure CE marking compliance and invest in robust clinical validation. France, Germany, and the United Kingdom remain key buyers of high-end forceps, whereas emerging markets in the Middle East and North Africa show potential for growth as surgical capacities expand. Healthcare systems across these diverse regions are navigating reimbursement policies and budget constraints while balancing the pursuit of advanced surgical outcomes.
Asia Pacific has emerged as both a significant consumer and a strategic manufacturing hub. Countries such as China and India are witnessing surges in elective surgeries, supported by rising medical tourism and hospital network expansions. Cost-sensitive markets in Southeast Asia drive innovation in lower-cost single-use instruments, whereas advanced centers in Japan and Australia continue to favor premium reusable forceps. This interplay of market maturity levels emphasizes the need for tailored regional strategies and agile supply chain solutions.
Assessing the Innovative Strategies and Competitive Positioning of Leading Manufacturers in the Titanium Micro Forceps Industry
The competitive landscape for titanium micro forceps features a combination of established global enterprises and agile innovators. Precision Surgical Instruments has distinguished itself through investments in automated manufacturing lines that enhance tip consistency and reduce lead times. NanoGrip Technologies has carved a niche by integrating nanocoatings that minimize tissue adhesion, while MediCraft Solutions has focused on modular handle systems that accommodate a range of tip geometries. Additionally, OmniMed Tools has forged partnerships with academic research centers to co-develop forceps optimized for emerging robotic-assisted interventions.Competitive positioning is increasingly defined by the breadth of product portfolios, the speed of new product introductions, and the depth of customer support services. Market leaders are expanding their footprints through strategic acquisitions that complement their core competencies, while smaller firms are securing advantage by focusing on specialized applications and rapid prototyping capabilities. Collaborative ventures between device manufacturers and original equipment producers have also become more prevalent, enabling the integration of forceps into multifunctional instrument platforms.
Given the intensifying focus on cost efficiency, many companies are exploring dual-use models that allow for both disposable and reusable forceps production. This approach caters to diverse customer segments and serves to hedge against fluctuations in sterilization protocols. Ultimately, sustained leadership will hinge on a firm's ability to innovate incrementally, maintain regulatory compliance, and cultivate strong relationships across the surgical community.
Strategic Recommendations for Industry Executives to Enhance Product Innovation, Supply Chain Resilience, and Market Penetration in Titanium Micro Forceps
To capitalize on emerging opportunities and navigate market complexities, industry leaders should pursue a multifaceted strategic roadmap. First, investing in additive manufacturing platforms will enable rapid customization of instrument geometries and support small-batch production runs for specialized clinical applications. Moreover, integrating digital quality control systems can enhance traceability and reduce nonconformance risks, driving greater confidence among procurement teams.Second, diversifying raw material sourcing and exploring nearshoring options will bolster supply chain resilience against tariff fluctuations and geopolitical disruptions. Establishing regional assembly hubs in key markets across the Americas and Asia Pacific can not only optimize logistics but also strengthen local partnerships. Third, companies should collaborate with regulatory bodies to anticipate evolving standards, streamlining approval pathways for new products while maintaining robust post-market surveillance protocols.
Finally, forging alliances with surgical training centers and healthcare institutions will facilitate real-world validation of instrument performance and accelerate clinician adoption. Developing continuing education programs and digital simulation tools can showcase forceps capabilities, reinforce brand loyalty, and underscore commitment to patient safety. By balancing innovation investments with operational agility, manufacturers can achieve sustainable growth and reinforce their leadership positions in the evolving titanium micro forceps market.
Elucidating the Comprehensive Research Methodology Incorporating Expert Interviews, Data Triangulation, and Rigorous Analytical Techniques for Market Clarity
The research underpinning this comprehensive report drew upon a rigorous methodology designed to ensure both depth and reliability of insights. Primary data collection involved in-depth interviews with key opinion leaders, including surgeons, procurement managers, and distribution executives, providing firsthand perspectives on clinical requirements, purchasing criteria, and emerging demand patterns. These qualitative inputs were complemented by secondary research conducted through peer-reviewed journals, regulatory filings, and industry whitepapers, enabling a thorough contextual understanding of technological and policy developments.Data triangulation techniques were employed to validate findings, cross-referencing information from proprietary interview transcripts, publicly available datasets, and specialized trade publications. A structured framework guided the analysis of segmentation parameters, ensuring that product type, application area, end user, and sales channel insights were aligned with observed market behaviors. Quantitative models and scenario analyses were applied to assess the potential impacts of external factors, such as tariff changes and regional economic fluctuations.
Throughout the process, a multi-stage quality assurance protocol was implemented, encompassing independent reviews by subject matter experts and consistency checks against established market benchmarks. This robust approach not only enhances the credibility of the conclusions but also equips stakeholders with actionable intelligence to inform strategic decision making and investment planning.
Synthesizing Key Findings and Future Outlook for Titanium Micro Forceps to Empower Stakeholder Decision Making and Innovation Roadmaps
The titanium micro forceps market stands at a pivotal juncture, shaped by advances in manufacturing technologies, evolving regulatory landscapes, and shifting supply chain dynamics. The introduction of new tariffs has catalyzed strategic realignment, prompting a renewed focus on local production and nearshoring as viable pathways to cost containment. Meanwhile, segmentation insights reveal that both customized instruments and application-specific designs are essential drivers of differentiation, catering to the nuanced requirements of dental, neurosurgical, ophthalmic, and plastic surgery procedures.Regionally, established markets in the Americas and EMEA continue to demand high-performance reusable forceps, while Asia Pacific's heterogeneous landscape underscores the need for flexible go-to-market approaches. Competitive intelligence highlights that companies investing in additive manufacturing, advanced coatings, and modular design will be best positioned to respond to these diverse market needs. Looking forward, sustainable practices-such as the development of reusable models with efficient sterilization workflows-will further influence procurement strategies and environmental objectives.
Ultimately, the market's future trajectory will depend on stakeholders' ability to integrate technological innovation with supply chain agility and regulatory foresight. By adopting the recommendations outlined herein, industry participants can navigate uncertainties, capitalize on emerging opportunities, and secure long-term growth in the dynamic field of titanium micro forceps.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Angled Tip
- Curved Tip
- Straight Tip
- Application
- Cardiovascular Surgery
- Dental Surgery
- Endodontic Surgery
- Implantology
- Periodontal Surgery
- Neurosurgery
- Spinal Surgery
- Tumor Resection
- Ophthalmic Surgery
- Cataract Surgery
- Corneal Surgery
- Glaucoma Surgery
- Plastic Surgery
- End User
- Ambulatory Surgery Centers
- Hospitals
- Research Institutes
- Specialty Clinics
- Sales Channel
- Direct Sales
- Distributors
- Online Retail
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- AgnThos AB
- B. Braun SE
- Accurate Surgical & Scientific Instruments corp.
- Ambler Surgical, LLC
- Avantor, Inc.
- Cairn Technology
- Daud Jee Mfg. Co.
- Harvard Bioscience, Inc.
- New Med Instruments
- Novo Surgical Inc.
- P.W. Coole & Son Ltd
- Precision Surgical Ltd
- RWD Life Science Co.,LTD
- Stille AB
- Surgical Tools, Inc.
- Teleflex Incorporated
- Wexler Surgical, Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Titanium Micro Forceps market report include:- AgnThos AB
- B. Braun SE
- Accurate Surgical & Scientific Instruments corp.
- Ambler Surgical, LLC
- Avantor, Inc.
- Cairn Technology
- Daud Jee Mfg. Co.
- Harvard Bioscience, Inc.
- New Med Instruments
- Novo Surgical Inc.
- P.W. Coole & Son Ltd
- Precision Surgical Ltd
- RWD Life Science Co.,LTD
- Stille AB
- Surgical Tools, Inc.
- Teleflex Incorporated
- Wexler Surgical, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
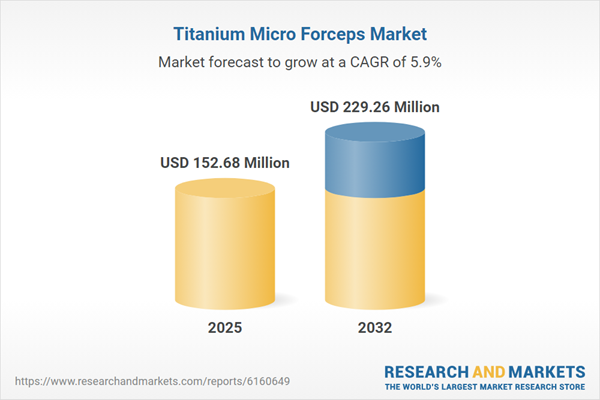
| Estimated Market Value ( USD | $ 152.68 Million |
| Forecasted Market Value ( USD | $ 229.26 Million |
| Compound Annual Growth Rate | 5.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 18 |