North America Generic Injectables Market Analysis
Generic injectables are drugs that are administered generally through intramuscular or intravenous routes. They are manufactured to meet the safety and efficacy profile of the brand-name drugs. Generic injectables are comparatively cheaper and are widely used by patients who need injections frequently. Several branded injectables are facing patent expiration which allows the entry of generic versions, contributing to the expansion of the global generic injectables market. Moreover, the rising advancements in the manufacturing processes of these generic drugs such as lyophilization and aseptic processing are enabling generic manufacturers to produce high-quality products, thereby driving the North America generic injectables market growth.Injectable drugs are known for their faster therapeutic effects as compared to oral drugs due to their higher bioavailability and thus are highly critical for emergency situations. The increasing burden of chronic diseases along with the growing geriatric population are a significant growth driver for this market. The American Cancer Society's annual cancer statistics report released in January 2024 estimated that new cancer cases will surpass 2 million in the United States in 2024, which translates to around 5,500 cancer diagnoses in a single day. The increasing prevalence of such diseases will fuel the need for effective medications including injectables, which are poised to augment the North America generic injectables market demand.
One of the major market trends is the surge in drug approvals of generic injectables by the health regulatory bodies. For instance, in May 2024, Indian-based generic injectable manufacturer Gland Pharma Limited announced the approval for its generic Edaravone injection of strengths 30mg/100ml and 60mg/100ml single-dose bags indicated for amyotrophic lateral sclerosis from the United States Food and Drug Administration (FDA). The injectable product, expected to be launched in 2025, is a generic equivalent of Mitsubishi Tanabe Pharma Corporation's Radicava injection, 30 mg/100 ml and 60 mg/100 ml. The rising introduction of such high-quality generic injectables, supported by the presence of a favorable regulatory environment, is anticipated to boost the market share.
North America Generic Injectables Market Segmentation
The report offers a detailed analysis of the market based on the following segments:Market Breakup by Product Type
- Large Molecule Injectables
- Small Molecule Injectables
Market Breakup by Container Type
- Vials
- Premix
- Prefilled Syringes
- Ampoules
- Others
Market Breakup by Application
- Oncology
- Cardiovascular
- CNS
- Infectious Diseases
- Autoimmune Disorders
- Others
Market Breakup by Route of Administration
- Intravenous
- Intramuscular
- Subcutaneous
- Others
Market Breakup by Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Others
Market Breakup by Region
- United States of America
- Canada
Leading Players in the North America Generic Injectables Market
The key features of the market report include patent analysis, grants analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:Pfizer Inc.
The global pharmaceutical giant Pfizer has a strong presence in the market through its subsidiary, Hospira. Hospira specializes in injectable drugs and infusion technologies and was acquired by Pfizer in 2015.Teva Pharmaceutical Industries Ltd.
Headquartered in Israel, Teva is one of the largest generic drug manufacturers in the world. The company is leveraging strategic acquisitions and its considerable manufacturing capacity to expand its presence in the generic injectables market.Baxter
Baxter International Inc. is a global leader in generic injectables, primarily serving areas such as anesthesia and critical care.Novartis Pharmaceuticals Corporation
Novartis Pharmaceuticals Corporation, through its spin-off Sandoz, is a key player in the generic injectables market, focusing on therapeutic areas such as oncology, immunology, and endocrinology.Other players in the market include Fresenius SE & Co. KGaA, Endo, Inc., Hikma Pharmaceuticals PLC, Dr. Reddy's Laboratories Ltd., Sagent Pharmaceuticals, Viatris Inc. (Mylan N.V.), Biocon, Sanofi, Lupin, and Aurobindo Pharma Limited, among others.
Kindly note that this only represents a partial list of companies, and the complete list has been provided in the report.
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Pfizer Inc.
- Teva Pharmaceutical Industries Ltd.
- Baxter
- Novartis Pharmaceuticals Corporation
- Fresenius SE & Co. KGaA
- Endo, Inc.
- Hikma Pharmaceuticals PLC
- Dr.Reddy’s Laboratories Ltd.
- Sagent Pharmaceuticals
- Viatris Inc.
- Biocon
- Sanofi
- Lupin
- Aurobindo Pharma Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
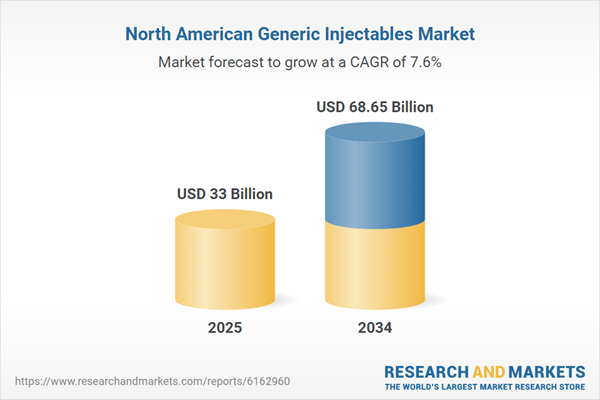
| Estimated Market Value ( USD | $ 33 Billion |
| Forecasted Market Value ( USD | $ 68.65 Billion |
| Compound Annual Growth Rate | 7.6% |
| Regions Covered | North America |
| No. of Companies Mentioned | 14 |