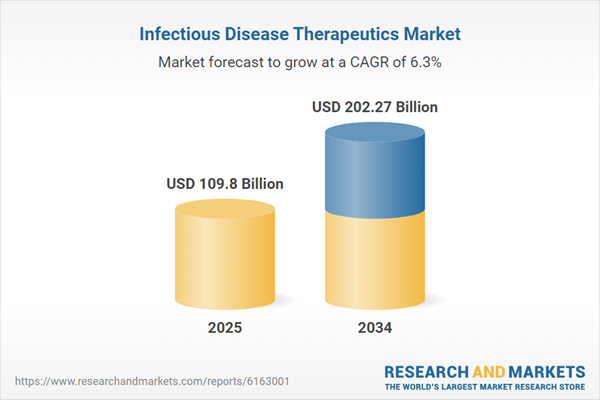
Global Infectious Disease Therapeutics Market Overview
Symptoms of suffering from an infectious disease include fever and fatigue but they may also vary depending upon the organism causing the infection. Mild infections may respond to rest and home remedies, while some life-threatening infections may need hospitalization. Many infectious diseases, such as measles and chickenpox, can be prevented by vaccines. Frequent and thorough handwashing also helps in protection against most infectious diseases.The infectious disease therapeutics market growth can be attributed to the increasing research and development activities initiated by the key players to develop and manufacture new and improved medicines that are expected to be more effective in targeting infectious diseases. The increasing launches of new medications in the market are also contributing to the market growth. Insurance companies are now providing attractive reimbursement policies to support the expenses of people, enabling them to opt for more suitable treatment options. With these reimbursement policies, people do not have to rethink their financial status at the time of needing suitable treatment for their infectious disease.
Additionally, the infectious disease therapeutics market demand is expected to expand further due to the increasing number of collaborations between key players, academic institutions, and medicinal institutions. These collaborations are targeted toward developing new product pipelines that will provide beneficial opportunities in the forecast period. Moreover, the market growth is anticipated to be driven by the rise in funding by the government and private sectors for the development of new infectious disease treatment approaches and medications.
Increasing Number of Clinical Trials
In August 2023, A team of University of Minnesota Medical School researchers successfully tested a new antifungal therapy to treat fungal meningitis. The research team tested a new oral formulation of the antifungal medication amphotericin among people who had HIV and cryptococcal meningitis, a common fungal infection around the brain. The new lipid nanocrystal formulation, which was tested in the EnACT trial can be taken orally and is non-toxic. In this randomized trial, 141 HIV-positive people with cryptococcal meningitis received oral amphotericin combined with oral flucytosine. Such developments are likely to contribute significantly to the infectious disease therapeutics market share.This combination was found to be promising for cryptococcal meningitis with antifungal activity, similar survival, and less toxicity in comparison to intravenous amphotericin B. Statistically fewer lab abnormalities occurred with the six weeks of LNC-enabled oral amphotericin B compared to one week of intravenous amphotericin B. In the group given two IV loading doses than the experimental oral amphotericin formulation, 90% of participants survived >4 months compared to 85% who received one week of standard intravenous amphotericin.
Cryptococcal meningitis is the most common cause of central nervous system infection in people living with HIV worldwide. Conventional amphotericin B can only be administered directly into veins and is highly toxic.
Being a less invasive and patient-friendly method of administration compared to the conventional intravenous route, this combination has a large potential for infectious disease therapeutics demand in the market. Additionally, better tolerability, fewer side effects, and improved overall patient safety indicate an advancement in the treatment of meningitis, propelling the market growth.
Rising Developments of New Vaccines in the Market
Fungal infections, causing a substantial number of deaths annually and leading to increased healthcare costs, represent a significant medical challenge. The lack of effective vaccines adds to the complexity of managing these infections. Fungal infections cause nearly 1.5 million deaths each year, and there is no vaccine to prevent them.A new vaccine from the University of Georgia has shown the potential to be the first clinically approved immunization to protect against invasive fungal infections, a growing concern as antifungal drug resistance increases.
The development of a new vaccine for preventing invasive fungal infections can be considered a significant advancement, which can significantly aid the infectious disease therapeutics market growth. The introduction of a clinically approved immunization from the University of Georgia that could protect against invasive fungal infections addresses a critical gap in current therapeutic options. If successful, this vaccine could potentially reduce the burden of fungal infections, improve patient outcomes, and contribute to overall public health.
Increased Fundings in R&D of New Treatments
Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) has served as a global non-profit partnership dedicated to accelerating antibacterial products to address drug-resistant bacteria, a leading cause of death around the world. In August 2023, the organization received significant funding from three governments to continue their work on breakthrough therapeutics, vaccines, and diagnostics. Contracts have been signed for CARB-X to secure funding from the UK government (USD 30.48 million over four years) and the German government (39 million euros over four years, and USD 49.53 for accelerator). The Canadian government has also announced its plan to support CARB-X with CAD USD 4.725 million over two years.CARB-X is a significant global initiative that addresses the challenge of drug-resistant bacteria. The UK, German, and Canadian governments have provided funding to support CARB-X in accelerating the development of antibacterial products, including vaccines, diagnostics, and therapeutics. These funds are likely to boost the infectious disease treatment market growth.
This financial support is crucial for CARB-X to continue its important work in researching and developing breakthrough solutions to combat antibiotic-resistant bacteria. Initiatives like this are essential for advancing the field of infectious disease therapeutics because drug-resistant bacteria pose a serious threat to public health worldwide.
By providing funding for CARB-X, governments are making a valuable contribution to the development of innovative and effective solutions to address this global health challenge.
Global Infectious Disease Therapeutics Market Segmentations
Infectious Disease Therapeutics Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Disease
- HIV/AIDS
- Influenza
- Hepatitis
- Tuberculosis
- Malaria
- Others
Market Breakup by Infection Type
- Bacterial
- Viral
- Fungal
- Parasitic
- Others
Market Breakup by Treatment Type
- Drugs
- Vaccines
Market Breakup by Mode of Treatment
- Oral
- Parenteral
- Topical
- Others
Market Breakup by Distribution Channel
- Hospitals
- Clinics
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Infectious Disease Therapeutics Market Regional Analysis
The increasing prevalence of infectious diseases across the globe is primarily driving the market growth. The rising geriatric population is also increasing the upcoming opportunities as aged people are much more susceptible to infectious diseases due to their weakened immune systems with age. The increasing consumption of antiviral drugs globally has also contributed to the market growth. With the increasing advancement of technologies, the more advanced and personalized medicines for infectious diseases have been propelling market growth as well.Additionally, this rise in disposable income is creating opportunities for key insurance companies to provide policies and plans with which people can avail suitable treatments for themselves without worrying about their finances. This rising disposable income is enabling more people to access optimal treatment approaches, further escalating the infectious disease therapeutics market growth. The sedentary lifestyle contributing to weakened immune systems over time is also contributing to the market growth.
In March 2023, The U.S. Food and Drug Administration (FDA) approved rezafungin for injection, (REZZAYOTM), a treatment for patients with candidemia and invasive candidiasis. The new treatment is the first approved for the invasive fungal infection in over a decade. It is a novel antifungal therapy given once a week intravenously.
The approval of rezafungin has the potential to fill a crucial gap of unmet medical needs for patients with invasive fungal infections, particularly candidemia. The introduction of new and innovative treatments is often positively received in the medical community, as it can offer alternative options for patients who may not have responded well to existing therapies, bolstering the market growth.
Infectious Disease Therapeutics Market: Competitor Landscape
In December 2023, Pfizer was granted a patent for pyridine derivatives that are substituted by heterocycle and amino groups. These compounds are antifungal agents and can be used for the treatment of fungal diseases and infections. The patent covers a specific compound with a defined chemical formula.The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:
- Pfizer Inc.
- GlaxoSmithKline plc.
- Merck & Co.
- F. Hoffmann-La Roche Ltd.
- AstraZeneca Plc
- Mylan N.V.
- Novartis AG
- Sanofi SA
- Takeda Pharmaceutical Company Limited
- Astellas Pharma
- AbbVie Inc.
- Boehringer Ingelheim GmbH
- Gilead Sciences
- Johnson & Johnson
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Pfizer Inc.
- GlaxoSmithKline plc.
- Merck & Co.
- F. Hoffmann-La Roche Ltd.
- AstraZeneca Plc
- Mylan N.V.
- Novartis AG
- Sanofi SA
- Takeda Pharmaceutical Company Limited
- Astellas Pharma
- AbbVie Inc.
- Boehringer Ingelheim GmbH
- Gilead Sciences
- Johnson & Johnson
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 109.8 Billion |
| Forecasted Market Value ( USD | $ 202.27 Billion |
| Compound Annual Growth Rate | 6.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 14 |