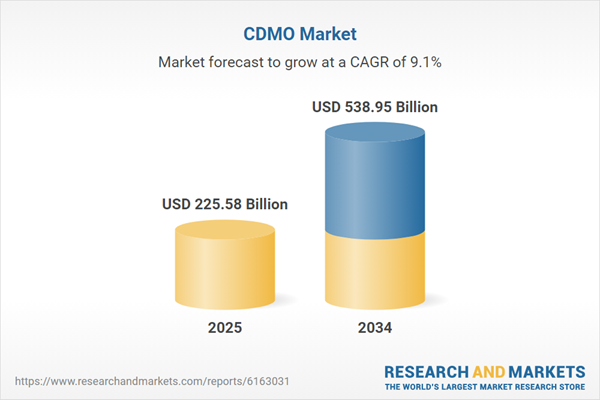
Global Contract Development and Manufacturing Organization (CDMO) Market Overview
Contract development and manufacturing organization (CDMO) refers to an organization that partners with pharmaceutical companies to provide drug development and manufacturing services on a contractual basis. CDMO can help these firms outsource the manufacturing of drugs as well as assist in the development activities involved before the manufacturing process. Partnering with a CDMO offers multiple benefits to the pharmaceutical company such as reduced infrastructure costs, expertise from skilled staff, and necessary manufacturing capacity to increase production.According to the data released by the World Health Organisation (WHO), chronic diseases or non-communicable diseases cause 41 million deaths every year, accounting for 74% of deaths globally. The prevalence of these chronic diseases, including cardiovascular diseases, cancer, and diabetes, responsible for increasing mortality rates, fuels the need for effective treatment options like novel drugs and therapies. Subsequently, this elevates the contract development and manufacturing organization (CDMO) market demand as more and more pharmaceutical companies are opting for such services to offer improved and efficient solutions to patients.
Increased Mergers and Acquisitions to Expedite Drug Development and Delivery
Contract development and manufacturing organizations (CDMOs) are increasingly engaging in mergers and acquisitions to keep up with changing customer requirements, which is a significant trend aiding market growth. In June 2023, KBI Biopharma, Inc., a cell line development CDMO, merged with Selexis SA, a global life sciences company, integrating as one organization under the name KBI Biopharma. This merger event will streamline drug development and manufacturing processes, including clinical and cGMP manufacturing services for mammalian programs.Strategic partnerships can reduce manufacturing risks and accelerate the commercialization process of drugs by employing integrated resources and solutions. In January 2024, Kindeva Drug Delivery (a global CDMO specializing in drug-device combination products), announced the acquisition of Summit Biosciences Inc. (an intranasal drug delivery CDMO in the United States), to enhance its drug delivery capabilities and expand its biopharma customer base. The acquisition aims to offer improved intranasal drug delivery options in the coming years.
Strategic Investments Reinforce Contract Development and Manufacturing Organization (CDMO) Market Growth and Expansion
In July 2023, a leading biopharmaceuticals CDMO, Biovian announced its decision to invest EUR 50 million to facilitate the expansion of its manufacturing unit in Finland. The facility will be constructed in an area of 69,000 sq. ft, harboring advanced technologies to aid the development of Advanced Therapy Medical Products (ATMP), including adeno-associated viral therapies. The investment will help in increasing the production capacity of the company to align with the growing size of the CDMO market.
To meet the rising market demand, CDMOs are rapidly expanding their manufacturing capacities and capabilities to deliver optimal services to their customers at a larger scale. Strategic investments have enabled the leading CDMO players in the market to adapt to the changing landscape in the biopharmaceutical industry.
Global Contract Development and Manufacturing Organization (CDMO) Market Segmentation
Contract Development and Manufacturing Organization (CDMO) Market Report and Forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Market Breakup by Service Type CMO:
- Active Pharmaceutical Ingredient (API) Manufacturing
- Small Molecule
- Large Molecule
- High Potency (HPAPI)
- Finished Dosage Formulation (FDF) Development and Manufacturing
- Solid Dose Formulation
- Liquid Dose Formulation
- Injectable Dose Formulation
- Secondary Packaging Services
Market Breakup by Research Phase CRO:
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
Market Breakup by Therapeutic Area:
- Oncological Diseases
- Cardiovascular Diseases
- Infectious Diseases
- Others
Market Breakup by Region:
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Contract Development and Manufacturing Organization (CDMO) Market Regional Analysis
North America has been leading the contract development and manufacturing organization (CDMO) market during the historical period. This can be attributed to the presence of top CDMO players in the region who are constantly making investments and taking mergers and acquisitions initiatives to upscale their drug manufacturing capacities. In January 2023, a United States-based CDMO, Agilent Technologies reported a USD 725 million investment to double its manufacturing capacity. The investment was made to accommodate the surge in the therapeutic nucleic acids market and develop drugs targeting prevalent diseases like cancer and cardiovascular diseases.Europe is another major market expected to hold a substantial contract development and manufacturing organization (CDMO) market share in the coming years. The robust medical infrastructure and advanced healthcare system drive the demand for novel drugs and vaccines to treat and prevent chronic diseases. Consequently, the major biopharmaceutical firms are working towards catering to the unmet medical needs of the patients.
Global Contract Development and Manufacturing Organization (CDMO) Market: Competitor Landscape
In January 2023, Lottee Biologics, a CDMO company headquartered in South Korea, announced its investment plan of USD 3 billion to build three mega plants, each with a production capacity of 360,000 liters by 2030. The company is expected to begin commercial production by 2027, aiming to generate USD 3 billion in revenue by 2034 and bolster the biopharma ecosystem, thereby accelerating the discovery of new therapeutics. The rising trend of internal investments by prominent biopharmaceutical companies is anticipated to increase the contract development and manufacturing organization (CDMO) market size in the forecast period.The key features of the market report include patent analysis, grants analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:
- Catalant Inc.
- Baxter Biopharma Solutions (Baxter International Inc.)
- Vetter Pharma-Fertigung GmbH & Co. KG
- Recipharm AB
- Albany Moleculer Research Inc. (AMRI)
- Thermo Fisher Scientific
- Boehringer Ingelheim Group
- Pfizer Inc.
- NextPharma Technologies
- Jubilant Pharmova Ltd
- Famar SA
- Lonza Group
- TapeMark
- Novotech Pty Ltd
- ARX LLC
- Aenova Holding GmBH
- Tesa Labtec GmbH (TESA SE)
- CMIC Holdings Company Ltd
- Syneos Health Inc.
- LabCorp Drug Development
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Catalant Inc.
- Baxter Biopharma Solutions (Baxter International Inc.)
- Vetter Pharma-Fertigung GmbH & Co. KG
- Recipharm AB
- Albany Moleculer Research Inc. (AMRI)
- Thermo Fisher Scientific
- Boehringer Ingelheim Group
- Pfizer Inc.
- NextPharma Technologies
- Jubilant Pharmova Ltd
- Famar SA
- Lonza Group
- TapeMark
- Novotech Pty Ltd
- ARX LLC
- Aenova Holding GmBH
- Tesa Labtec GmbH (TESA SE)
- CMIC Holdings Company Ltd
- Syneos Health Inc.
- LabCorp Drug Development
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 225.58 Billion |
| Forecasted Market Value ( USD | $ 538.95 Billion |
| Compound Annual Growth Rate | 9.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |