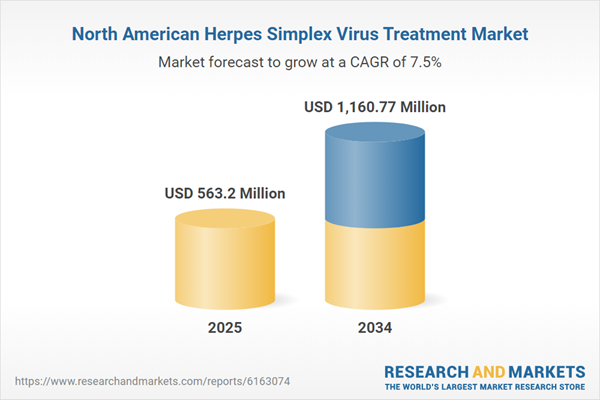
North America Herpes Simplex Virus Treatment Market Analysis
Herpes simplex virus (HSV) or herpes is a treatable infection that can cause painful ulcers or blisters. Type 1 (HSV-1) and Type 2 (HSV-2) are two types of herpes simplex virus. Anti-HSV medications such as acyclovir, famciclovir, and valacyclovir, are generally prescribed to prevent the virus from multiplying. Oral pain relievers (paracetamol or ibuprofen) and topical pain relievers (benzocaine, L-lysine, and docosanol) are often taken to manage pain. Heightened patient awareness surrounding herpes is leading to higher diagnosis rates, which contributes to the expansion of the global herpes simplex virus treatment market. Further, the increasing focus on the development of innovative treatments such as gene therapy to permanently cure the infection is expected to drive the North America herpes simplex virus treatment market growth.In the United States, herpes simplex virus (HSV) 1 and 2 are considered the most common viral infections. Around 80% of individuals between the ages of 14 and 49 are affected by HSV-1 and over 10% are infected with HSV-2. To date, there are no HSV vaccines approved by the US Food and Drug Administration and current treatment strategies are ineffective against latent HSV infections. To accelerate the development of vaccines, therapeutics, and diagnostics against herpes, the National Institutes of Health (NIH) established the Strategic Plan for Herpes Simplex Virus Research 2023-2028, aligning with the Sexually Transmitted Infections (STI) National Strategic Plan. The NIH HSV research plan states 4 priorities: increase knowledge of HSV biology, improve diagnosis, enhance treatment strategies, and advance research to prevent this viral infection. Such initiatives are likely to expedite the introduction of novel interventions, which will directly impact the North America herpes simplex virus treatment market demand.
One of the major market trends is the rising funding for herpes simplex virus research with the aim of reducing the associated health consequences. In October 2023, infectious disease company, Rational Vaccines, Inc. (RVx) announced that it had received USD 2.8 million in funding from the National Institute of Health (NIH) to boost its research in the treatment, diagnosis, and prevention of the herpes simplex virus infection. The company was awarded three separate grants to further its development of highly sensitive HSV-type-specific tests, live-attenuated HSV-1 strain ‘VC2' to prevent and treat ocular herpes, and prophylactic and therapeutic HSV vaccines to curb the spread of herpes. Increased investment to stimulate advancements in herpes simplex virus testing and treatments is poised to augment the market share.
North America Herpes Simplex Virus Treatment Market Segmentation
The report titled “North America Herpes Simplex Virus Treatment Market Report and Forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Breakup by Type
- Herpes Simples Virus-1 Infection
- Herpes Simplex Virus-2 Infection
- Others
Breakup by Drug Type
- Acyclovir
- Valacyclovir
- Famciclovir
- Others
Breakup by Route of Administration
- Oral
- Parenteral
- Topical
Breakup by Region
- United States
- Canada
Leading Players in the North America Herpes Simplex Virus Treatment Market
The key features of the market report include clinical trials analysis, grants analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:Roche Holding AG
F. Hoffmann-La Roche AG, a global pioneer in pharmaceuticals and diagnostics, is actively involved in developing serology and molecular assays to provide testing and result interpretation of herpes simplex virus infections.AbbVie Inc.
The global biopharmaceutical company AbbVie Inc. is involved in the clinical development of treatments intended for combating viral infections.Johnson & Johnson
Johnson & Johnson Innovative Medicine is known for heavily investing in its research initiatives to develop therapeutics and vaccines for the treatment and prevention of life-threatening infectious diseases.GlaxoSmithKline plc
GlaxoSmithKline plc, a British multinational pharmaceutical company headquartered in London, is conducting a clinical trial to evaluate its targeted immunotherapy (GSK3943104A) efficacy against HSV infections.Other players in the market include Sanofi, Mylan N.V., Teva Pharmaceutical Industries Ltd., Novartis AG, Merck & Co., Inc., and Pfizer Inc.
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Roche Holding AG
- AbbVie Inc.
- Johnson & Johnson
- GlaxoSmithKline plc
- Sanofi
- Mylan N.V.
- Teva Pharmaceutical Industries Ltd.
- Novartis AG
- Merck & Co., Inc.
- Pfizer Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 563.2 Million |
| Forecasted Market Value ( USD | $ 1160.77 Million |
| Compound Annual Growth Rate | 7.5% |
| Regions Covered | North America |
| No. of Companies Mentioned | 10 |