Oncology Based In-Vivo CRO: Introduction
Oncology based in-vivo CRO provides specialized services in the field of oncology research. A diverse range of services is provided by this organization in order to support the development of new oncology therapies and treatments. The main services provided by Oncology based In-Vivo CRO are study design, selection and validation of animal model, data collection and analysis, and regulatory compliance, along with dosing and drug administration, and tumor imaging and measurement.The specialized CROs provide knowledge and experience in the conduct of investigations and tests employing in vivo models, such as animal models, to assess the efficacy and safety of new oncology treatments. Through the use of in vivo tumor models, xenograft and syngeneic models, pharmacokinetic investigations, toxicological analyses, and efficacy studies, in-vivo CROs offer thorough evaluation of potential oncology medications. They aid in finding indicators of therapy response or resistance, helping to test novel cancer therapies, and analyzing how treatments affect tumor growth and metastasis.
Global Oncology based In-Vivo CRO Market Analysis
Expert services are provided by these organizations due to the access they have to advanced technologies and equipment that are required and necessary to conduct in-vivo studies. The expansion of personalized medicine owing to the market growth. By offering preclinical services that promote the development of targeted medicines and ease the discovery and validation of biomarkers, in-vivo CROs are significantly contributing to the advancement of personalized medicine. Additionally, the field of immuno-oncology harnesses the body's immune system to fight cancer has also been witnessing significant growth, leading to further oncology based in-vivo CRO market expansion.In-Vivo CROs actively conducting preclinical studies to evaluate the efficacy and safety of immunotherapies and combination therapies to support the immuno-oncology research, propelling the oncology based in-vivo CRO market growth. The increased integration of artificial intelligence and machine learning technologies to improve the quality of data analysis, image processing, and predictive modeling are directly contributing to the market growth. In-Vivo CROs are the technologies that enables better interpretation of complex preclinical data and facilitate the identification of novel biomarkers, improving decision-making processes and accelerating drug discovery efforts.
Global Oncology based In-Vivo CRO Market Segmentation
“Oncology Based In-Vivo CRO Market Report and Forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Market Breakup by Indication
- Blood Cancer
- Solid Tumors
- Other Indications
Market Breakup by Model
- Syngeneic
- Xenograft
- Patient Derived Xenograft (PDX)
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Oncology based In-Vivo CRO Market Overview
The market for oncology based in-vivo CRO has been witnessing evident growth in the past and is expected to see the growth in coming years as well owing to the increasing prevalence of cancer worldwide. The demand for newly developed therapies that are effective and innovative has increased and has created an emphasis on research and development for these therapies in the oncology filed. The demand for cost-effective and time-efficient drug development procedures has also been a driver for market growth.In-vivo CROs provide specialised knowledge in creating and using animal models that closely resemble the characteristics of human cancers, enabling the assessment of targeted treatments and individualised treatment plans. This is further aiding the oncology based in-vivo CRO market development.
To ensure that only the highest-quality products are let onto the market, health authorities have established strict regulatory restrictions on pharmaceutical businesses. In-Vivo CROs help these companies adhere to these regulations. In-Vivo CROs assist in ensuring that potential oncology treatments meet the requisite safety and efficacy requirements for further development and clinical trials by conducting preclinical research in accordance with Good Laboratory Practise (GLP) standards which directly contributes to the market growth.
Oncology based In-Vivo CRO Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Crown Bioscience Inc.
- Charles River Laboratories Inc.
- ICON PLC
- Taconic Biosciences Inc.
- Covance Inc.
- Eurofins Scientific
- EVOTEC
- The Jackson Laboratory
- Wuxi AppTec.
- MI Bioresearch Inc.
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Crown Bioscience Inc.
- Charles River Laboratories Inc.
- ICON PLC
- Taconic Biosciences Inc.
- Covance Inc.
- Eurofins Scientific
- EVOTEC
- The Jackson Laboratory
- Wuxi AppTec.
- MI Bioresearch Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
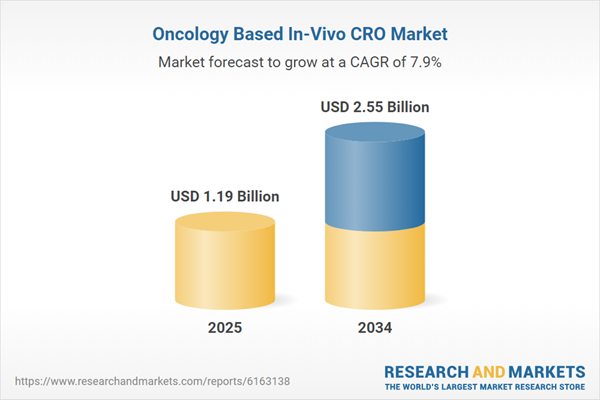
| Estimated Market Value ( USD | $ 1.19 Billion |
| Forecasted Market Value ( USD | $ 2.55 Billion |
| Compound Annual Growth Rate | 7.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |