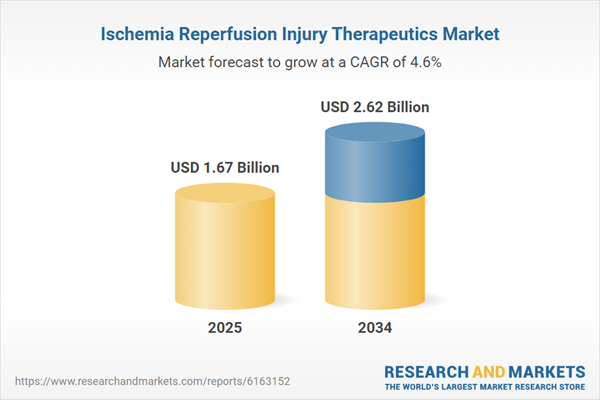
Ischemia Reperfusion Injury Therapeutics: Introduction
Ischemia reperfusion injury, also known as reperfusion injury or reoxygenation injury, occurs when blood supply returns to an ischemic area, leading to sudden tissue death. Instead of facilitating normal function in the cell, the restoration of oxygen and nutrients leads to inflammation and oxidative damage.Ischemia Reperfusion Injury Therapeutics Market Analysis
Ischemia-reperfusion injury is a major cause of multi-organ malfunction post a severe trauma with heavy bleeding. IRI occurrence may also lead to severe cardiac, pulmonary, spinal cord renal, hepatic and limb problems. Hence, the ischemia-reperfusion injury therapeutics market demand has increased.Metformin, a plant-based anti-hyperglycemic drug having multiple mechanisms of action, has been a common ischemia reperfusion injury drug. It modulates ROS (reactive oxygen species) levels and oxidative damage during reperfusion, preventing organ malfunction. Focusing on kidney transplantation, Treprostinil (Remodulin) is another antihypertensive agent which is formulated as an injectable solution, also being considered for the treatment of ischemia-reperfusion injury.
With the emergence of nanotechnology in the medical field and a better understanding of cell interactions, scientists have diverted towards applying nanoparticle-mediated medicine. It can be considered an effective therapy owing to its enhanced drug accumulation, reduced systemic toxicity and improved pharmacokinetics. Numerous biomaterials like microneedles, liposomes, cardiac patches, and hydrogels are being explored as a potential tool for treatment. Given the substantial research efforts dedicated to this area, the ischemia-reperfusion injury therapeutics market value is estimated to rise significantly during the forecast period.
Ischemia Reperfusion Injury Therapeutics Market Segmentation
Ischemia Reperfusion Injury Therapeutics Market Report and Forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Market Breakup by Treatment Type
- Drug Therapy
- Antioxidants
- Anti-Inflammatory Agents
- Vasodilators
- Immunomodulators
- Others
- Medicated Gases
- Others
Market Breakup by Indications
- Heart Injury
- Kidney Injury
- Intestine Injury
- Other Injury
Market Breakup by Route of Administration
- Oral
- Parenteral
- Topical
- Inhalation
Market Breakup by End User
- Hospitals and Clinics
- Ambulatory Surgical Centres
- Research Institutes
- Others
Market Breakup by Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
Market Breakup by Region- 7MM
- United States
- EU-4 and the United Kingdom
- Japan
Ischemia Reperfusion Injury Therapeutics Market Overview
Cardiovascular diseases continue to be some of the most chronic diseases worldwide, still affecting the lives of millions. Myocardial ischemia-reperfusion accounts for approximately 1.72% of deaths and disabilities in the global population. With the prevalence of cardiovascular, neurological, and kidney-related diseases, North America has held a significant part of the ischemia-reperfusion injury therapeutics market share in the historic period. A technologically advanced infrastructure, rising research and developments in medical care facilities and capital investments are key factors accountable for the market size.The Asia Pacific region is expected to witness exponential growth in the coming years owing to the presence of a geriatric population that may lead to an increased incidence of chronic diseases like myocardial infarction. Moreover, the government initiatives to build technology-supportive infrastructure, an influx of foreign funds due to the easy availability of resources and the emergence of healthcare startups, can bring some substantial innovations in the ischemia reperfusion injury therapeutics market.
Ischemia Reperfusion Injury Therapeutics Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Pfizer Inc.
- AstraZeneca PLC
- Novartis International AG
- Merck & Co., Inc.
- Johnson & Johnson
- Bayer AG
- Sanofi SA
- GlaxoSmithKline plc
- Boehringer Ingelheim International GmbH
- Bristol-Myers Squibb Company
- AbbVie Inc.
- Amgen Inc.
- Gilead Sciences, Inc.
- F. Hoffmann-La Roche Ltd.
- Eli Lilly and Company
- Takeda Pharmaceutical Company Limited
- Biogen Inc.
- Regeneron Pharmaceuticals, Inc.
- Mitsubishi Tanabe Pharma Corporation
- Grifols, S.A.
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Pfizer Inc.
- AstraZeneca PLC
- Novartis International AG
- Merck & Co., Inc.
- Johnson & Johnson
- Bayer AG
- Sanofi SA
- GlaxoSmithKline plc
- Boehringer Ingelheim International GmbH
- Bristol-Myers Squibb Company
- AbbVie Inc.
- Amgen Inc.
- Gilead Sciences, Inc.
- F. Hoffmann-La Roche Ltd.
- Eli Lilly and Company
- Takeda Pharmaceutical Company Limited
- Biogen Inc.
- Regeneron Pharmaceuticals, Inc.
- Mitsubishi Tanabe Pharma Corporation
- Grifols, S.A.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 1.67 Billion |
| Forecasted Market Value ( USD | $ 2.62 Billion |
| Compound Annual Growth Rate | 4.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |