Aortic Valve Replacement Devices Market Growth
To accelerate the adoption of aortic valve replacement devices in underdeveloped areas, stakeholders in the healthcare segment are focusing on improving the access of the population to interventional cardiology and cardiothoracic surgery specialities. In addition, a rise in educational campaigns to spread awareness regarding aortic valve diseases will increase the use of these devices in the treatment procedure.Figure: World Population of the Elderly (In Million)
As the number of elderly patients with aortic valve stenosis and various other comorbidities is growing, the adoption of less invasive therapies is increasing, as surgeries pose a significant risk to their life. Additionally, the expanding geriatric population in Asian countries is also expected to enhance the adoption of minimally invasive aortic valve replacement procedures.
Key Trends and Developments
Increasing aortic stenosis in geriatric population; adoption of advanced aortic valves to ease surgical procedures; rising sedentary lifestyles; and increasing cases of valvular heart diseases are impacting the aortic valve replacement devices market growthNov 9, 2022
Boston Scientific Corporation announced the launch of the LUX-Dx™ Insertable Cardiac Monitor (ICM) System, a long-term diagnostic device that is designed with a dual-stage algorithm and inserted under the patients' skin for the detection and verification of arrhythmias associated with conditions such as atrial fibrillation (AF), cryptogenic stroke and syncope.
Sep 12, 2022
Edwards Lifesciences launched its next-gen Sapien 3 Ultra Resilia transcatheter heart valve, after its FDA approval. The latest TAVR device incorporates Resilia tissue technology that provides enhanced calcium blocking properties and dry tissue packaging conditions for facilitating the ease of usage.
Feb 15, 2022
Boston Scientific Corporation closed the acquisition of Baylis Medical Company Inc., a company offering guidewires, sheaths, dilators for supporting catheter-based left-heart procedures and advanced transseptal access solutions.
Jan 10, 2022
Medtronic announced that the National Medical Products Administration (NMPA) approved the CoreValve™ Evolut™ PRO TAVR system to treat severe aortic stenosis (AS) for symptomatic patients in China who are at high or extreme risk for open heart surgery.
Rising cases of aortic stenosis in geriatric population
Aortic stenosis is a common life-threatening heart valve disease that can result in heart failure. Geriatric population can depict severe, symptomatic aortic stenosis.Advanced aortic valves to ease surgical procedures
Advanced aortic valves such as the sutureless aortic valve replacement (SU-AVR) and rapid deployment aortic valve replacement (RD-AVR) allow hospitals to conduct easy, effective, and safe valve implantation under direct vision.Rising sedentary lifestyles and chronic diseases
The prevalence of chronic diseases, such as hypertension, cholesterol and diabetes, owing to people's unhealthy and hectic lifestyle is increasing the risks of aortic valve failure and the need to replace it.Increasing use in the treatment of various aortic valve diseases
Aortic valve replacement devices are used to treat conditions including aortic valve regurgitation, aortic valve stenosis, and other congenital heart defects that affect the aortic valve.Aortic Valve Replacement Devices Market Trends
Aortic valve stenosis cases are increasing in numbers around the globe with a growing geriatric population and the prevalence of atherosclerosis. The geriatric population is highly susceptible to aortic valve conditions, particularly if they have cardiovascular risk factors such as high cholesterol and a history of smoking, which increase their chances for developing aortic valve calcification, requiring valve replacement. Patients aged 75 and above tend to have symptomatic and severe aortic stenosis and are investing in minimally invasive aortic valve replacement procedures to reduce their symptoms.People aged 75 and above are inclined towards less invasive transcatheter aortic valve replacements as conducting an open-heart surgery on them is risky as they are weak and may be suffering from multiple other diseases.
Additionally, procedures such as the SU-AVR, minimally invasive aortic valve replacement (MIAVR), and transcatheter aortic valve implantation/transcatheter aortic valve replacement (TAVI/TAVR) are being used to overcome the shortcomings of surgical AVRs.
Heart defects such as aortic valves with missing valve openings, incorrect valve size or shape, or blood flowing backwards are increasing the need for aortic valve replacement devices. Some unhealthy habits include a sedentary lifestyle with little to no exercise, alcohol consumption beyond safety limits, excessive stress damaging heart valves, and having a diet high in salt.
Aortic Valve Replacement Devices Industry Segmentation
“Global Aortic Valve Replacement Devices Market Report and Forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Market Breakup by Surgery Type:
- Open Surgery
- Minimally Invasive Surgery
Market Breakup by Product Type:
- Transcatheter Aortic Valve
- Sutureless Aortic Valve Replacement (SUAVR)
Market Breakup by End Use:
- Hospitals
- Ambulatory Surgical Centres
- Others
Market Breakup by Region:
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Aortic Valve Replacement Devices Market Share
Based on surgery type, minimally invasive surgery accounts for a significant share of the market
Minimally invasive aortic valve replacement surgeries are preferred by people and healthcare professionals as they prevent excess blood loss during the surgery, involve a lower risk of infection, and leave relatively fewer scars on the patient's body as they involve smaller chest incisions compared to open-heart surgery.Minimally invasive heart surgeries are associated with benefits like a shorter hospital stay, and faster recovery, and are less painful than the traditional methods.
Open-heart aortic valve replacement surgeries are advisable for patients who need to undergo multiple coronary bypass processes or complex cardiovascular operations.
Based on product type, TAVR significantly contributes to the global aortic valve replacement devices market revenue
The enhanced safety and fast recovery times offered by TAVR (transcatheter aortic valve replacement) have boosted its utilisation as an effective treatment for severe aortic stenosis among geriatric patients. Innovations in the design of valve prosthesis and technological advancements in its haemodynamic performance, along with the availability of data based on trials to establish the efficacy of TAVR, are expected to widen the scope of TAVR in low-risk and immediate patient groups.
Leading Companies in the Aortic Valve Replacement Devices Market
Market players are focusing on improving the availability of medical devices and delivering products that guarantee superior clinical outcomesBoston Scientific Corporation
Headquartered in the United States, Boston Scientific Corporation is a manufacturer and marketer of medical devices that are used in a broad range of interventional medical specialties. The company's core businesses are organized into three segments: Medsurg, Rhythm and Neuro, and Cardiovascular.Corcym S.r.l.
An Italian company, Corcym is primarily engaged in the manufacture of medical devices for heart surgeries. The company was formed after the acquisition of the LivaNova PLC (LivaNova) heart valve business by Gyrus Capital (Gyrus) in June 2021.Edwards Lifesciences Corporation
Edwards Lifesciences Corporation is primarily engaged in the manufacture of heart valve systems and repair products. The company manufactures transcatheter aortic valve replacement, transcatheter mitral and tricuspid technologies, and structural surgical heart products primarily in the United States (California and Utah), Singapore, Costa Rica, and Ireland.Medtronic plc
Headquartered in Ireland, Medtronic is a global leader in the healthcare technology sector. The company is engaged in the manufacture, distribution, and sales of device-based medical therapies and services in the cardiovascular, medical-surgical, neuroscience, and diabetes operating segments.Other notable players operating in the global aortic valve replacement devices market are Artivion, Inc, and Abbott Laboratories, among others.
Aortic Valve Replacement Devices Market Analysis by Region
North America is a key market for aortic valve replacement devices, with the US leading the way
North America remains a significant market, supported by the presence of advanced medical facilities, well-established healthcare infrastructure, and increasing geriatric population. In the United States, heart disease is the major cause of death for both men and women, and aortic stenosis, in particular, is one of the most common heart valve illnesses, affecting Americans over the age of 75 years. While surgical aortic valve replacement/implantation (SAVR, or SAVI) is the standard treatment for patients with severe aortic stenosis, transcatheter aortic valve replacement/implantation (TAVR, or TAVI) has established as a reliable and robust alternative for elderly patients who are at high risk for open surgery due to age, frailty, and other factors.Meanwhile, the growing geriatric population and the rising incidences of aortic stenosis in the Asia Pacific have bolstered the use of TAVR in clinical practices owing to its minimally invasive procedure, fast recovery times, and low mortality rates.
The Japanese government has been providing grants for innovative sutureless aortic heart valve, and unlike various other Asian countries, the national government also funds TAVI, enhancing the domestic accessibility to versatile aortic valve replacement devices.
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Boston Scientific Corporation
- Corcym S.r.l
- Edwards Lifesciences Corporation
- Medtronic plc
- Artivion, Inc.
- Abbott Laboratories
- Others
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
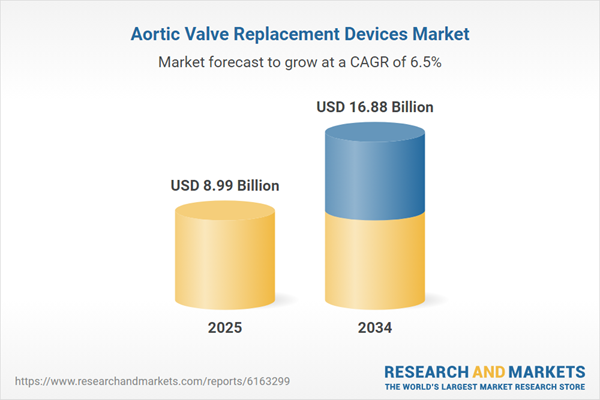
| Estimated Market Value ( USD | $ 8.99 Billion |
| Forecasted Market Value ( USD | $ 16.88 Billion |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 7 |