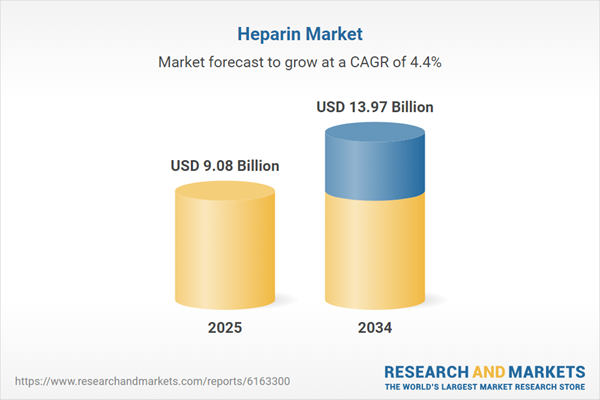
Global Heparin Market Overview
Heparin is an anticoagulant used to treat and prevent the formation of blood clots. Heparin cannot be absorbed via the digestive system, so it is taken intravenously or injected below the skin. Heparin drugs work by making thrombin (a coagulation factor) inactive, preventing clot formation in the blood. Heparin is used for certain medical conditions such as venous thromboembolism (VTE), deep vein thrombosis (DVT), heart attack, or after a medical procedure like heart surgery.The heparin market growth is driven by the increasing prevalence of cardiovascular diseases, which is poised to fuel the demand for anticoagulant products. Venous thromboembolism (VTE), characterized by the formation of a blood clot in the veins, and affects 1 in 12 individuals in the western region, with 20% of affected individuals dying within an year of diagnosis. As a result, there is a high demand for anticoagulants and is expected to increase in the forecast period. In addition, growth in healthcare expenditure and rising patient awareness are some of the key factors poised to accelerate the market growth in coming years.
Increased Approval for Heparin Drugs by Regulatory Bodies
The surge in drug approvals by health authorities such as the United States Food and Drug Administration (FDA) along with the increased use of heparin in various drug formulations is projected to increase the heparin market share in the coming years. In November 2023, the FDA approved DefenCath, a formulation of taurolidine and heparin, developed by a United States-based biopharmaceutical company CorMedix Inc. The therapeutic product DefenCath is an antimicrobial catheter lock solution that reduces catheter-related bloodstream infections (CRBSIs) by 71% in adult hemodialysis patients. The approval is based on the positive results from the phase 3 LOCK IT-100 trial.Rise in Product Launches to Meet the Rising Heparin Market Demand
In June 2023, Techdow USA Inc., a pharmaceutical company announced the launch of generic Enoxaparin Sodium (Preservative Free) Prefilled Syringes, a low molecular weight heparin (LMWH). Available at different strengths such as 30mg, 100mg, and 150mg, the drug prevents the formation of blood clots in various medical conditions. The U.S. FDA granted the final approval of the Abbreviated New Drug Application for the product as well. The commercial release and approval of such products indicate the commitment of the key market players to meet the rising demand for heparin.Global Heparin Market Segmentation
The report titled “Heparin Market Report and Forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Market Breakup by Products
- Unfractionated Heparin
- Low Molecular Weight Heparin (LMWH)
- Ultra-Low Molecular Weight Heparin (ULMWH)
Market Breakup by Source
- Bovine
- Porcine
Market Breakup by Applications
- Deep Vein Thrombosis (DVT)
- Atrial Fibrillation and Heart Attack
- Coronary Artery Disease
- Other Applications
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Heparin Market Regional Analysis
North America accounts for the largest share of the heparin market which can be attributed to the increasing prevalence of cardiovascular diseases in the region. According to the data released by the American Heart Association, cardiovascular diseases stand as the leading cause of death in the United States. Heparin, as an anticoagulant, plays a crucial role in treating and managing cardiovascular conditions and thus, has a significant demand in the region. In addition, the growth of the market is propelled by the presence of the key market players and robust support from the government.Asia Pacific also holds a significant heparin market share and is anticipated to witness rapid growth in the coming years. The major key drivers are the rising patient awareness and disease burden. Furthermore, technological advancements and intensive research and development activities to improve the healthcare system are expected to accelerate the market growth. In May 2023, a team of researchers at the Thailand Excellence Center for Tissue Engineering and Stem Cells, Department of Biochemistry from Chiang Mai University discovered an alternative source of heparin. They extracted acharan sulfate (heparin-like substances) from the mucus of the giant African snail, Achatina fulica, which has the potential to address the rising demand for the essential anticoagulant.
Global Heparin Market: Competitive Landscape
In November 2023, a multinational Danish pharmaceutical company, Leo Pharma, announced a cooperation agreement with a Greek pharmaceutical company, Vianex SA. Under the terms of agreement, Vianex will be responsible for manufacturing low molecular weight heparin and unfractionated heparin. On the other hand, Leo Pharma will handle the distribution of the final product. The production of five product codes is estimated to amount to several million vials per year. The objective of the collaboration is to increase their market presence and expand the distribution of their products in over 23 countries. This indicates a market trend of increasing collaborations among the leading market players to boost their manufacturing capacity. Consequently, this will fuel the heparin market share in the forecast period.The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:
- Aspen Pharmacare Holdings Limited
- B Braun Melsungen AG
- Baxter International
- Dr. Reddy's Laboratories
- Hebei Changshan Biochemical Pharmaceutical
- Leo Pharma AS
- Opocrin SpA
- Pfizer Inc
- Techdow USA
- Viatris Inc.
- Novartis AG
- Aspen Oss
- Leo Pharma A/S
- Hikma Pharmaceuticals PLC
- Sanofi
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Aspen Pharmacare Holdings Limited
- B Braun Melsungen AG
- Baxter International
- Dr. Reddy's Laboratories
- Hebei Changshan Biochemical Pharmaceutical
- Leo Pharma AS
- Opocrin SpA
- Pfizer Inc
- Techdow USA
- Viatris Inc.
- Novartis AG
- Aspen Oss
- Leo Pharma A/S
- Hikma Pharmaceuticals PLC
- Sanofi
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 9.08 Billion |
| Forecasted Market Value ( USD | $ 13.97 Billion |
| Compound Annual Growth Rate | 4.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |