Ambulatory Cardiac Monitoring Devices: Introduction
Ambulatory electrical monitors are used to record the electrical activity in the heart over a long period. These devices are wearable and convenient, allowing the patient to wear them easily. They can be worn for days, weeks, or even years. They can be used to diagnose problems that may happen unpredictably even if the patient is not under the supervision of a healthcare professional.Ambulatory monitors can diagnose arrhythmias, which are irregular heart rhythms. Some common types of arrhythmias include bradyarrhythmias, tachyarrhythmias, supraventricular arrhythmias, and ventricular arrhythmias.
Global Ambulatory Cardiac Monitoring Devices Market Analysis
The growth of the global market for ambulatory cardiac monitoring devices is majorly driven by the rising geriatric population, which is both an opportunity and a challenge. The rising cardiovascular disorders across the globe are also driving the market growth. The rise in cardiovascular disorder cases and the increasing geriatric population are majorly contributing to the increasing demand for ambulatory cardiac monitoring devices in the market.The increasing technological advancements are also gaining traction from healthcare professionals and facilities that are interested in providing the best services to the patient when it comes to cardiac care. Such developments are rapidly aiding the global ambulatory cardiac monitoring devices market growth. The integration of advanced technologies, such as artificial intelligence and machine learning in the healthcare sector, such as ambulatory monitoring devices, greatly influence the market growth as this integration brings clinical confidence and increases efficiency.
The increasing technological development in the market resulting from the increased number of clinical studies to improve the functionality of existing monitoring devices or developing new ones from scratch is a major trend intensifying the market growth. For example, the use of Philips MCOT (Mobile Cardiac Outpatient Telemetry) solutions, based on prescriptions, may result in less expenditure and better patient outcomes by monitoring for arrhythmic disturbances and promoting timely interventions, propelling the market growth. The introduction of smart devices with the integration of advanced technologies, such as machine learning and artificial intelligence, are also driving the growth of the global ambulatory cardiac monitoring devices market.
The increasing number of clinical trials to develop new devices and technologies for better results are also expected to drive the market growth in the coming years.
Global Ambulatory Cardiac Monitoring Devices Market Segmentations
Ambulatory Cardiac Monitoring Devices Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Type
- ECG Devices
- Holter Monitors
- Event Monitors
- Implantable Loop Recorders
- Mobile Cardiac Telemetry
Market Breakup by End User
- Hospitals and Clinics
- Ambulatory Care Centers
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Ambulatory Cardiac Monitoring Devices Market Overview
The growth of the global market is driven by the increasing geriatric population as older people are more susceptible to health conditions, such as high blood pressure, heart failure, diabetes, and thyroid disease, that may lead to arrhythmias. Arrhythmias caused by congenital heart defects or inherited conditions are common in children and young adults.Key players in the market and governments are greatly investing in research and development activities associated with the development of newly advanced ambulatory cardiac monitoring devices to improve results and patient experiences, which is further driving the global ambulatory cardiac monitoring devices market growth. The presence of robust reimbursement and regulatory policies in developed and emerging economies is expected to directly contribute to the growth of the market during the forecast period. Studies say that nearly 80% of premature heart attacks and strokes are preventable if diagnosed timely. The increasing number of research and clinical studies to overcome the increasing prevalence of cardiovascular diseases and disorders are driving the market growth.
Global Ambulatory Cardiac Monitoring Devices Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Abbott
- Boston Scientific Corporation
- GE Healthcare
- Cardinal Health
- Hill-Rom Holdings
- Koninklijke Philips N.V.
- Medtronic
- Nihon Kohden
- CorporationAsahi Kasei Corporation
- BIOTRONIK SE & Co KG
- BPL Medical Technologies
- iRhythm Technologies, Inc.
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Abbott
- Boston Scientific Corporation
- GE Healthcare
- Cardinal Health
- Hill-Rom Holdings
- Koninklijke Philips N.V.
- Medtronic
- Nihon Kohden Corporation
- Asahi Kasei Corporation
- BIOTRONIK SE & Co KG
- BPL Medical Technologies
- iRhythm Technologies, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
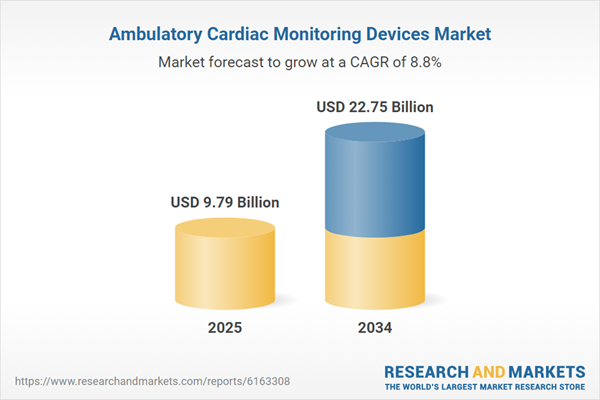
| Estimated Market Value ( USD | $ 9.79 Billion |
| Forecasted Market Value ( USD | $ 22.75 Billion |
| Compound Annual Growth Rate | 8.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |