Europe Generic Injectables Market Analysis
Generic injectables have the same active pharmaceutical ingredient as their brand-name injectables along with equivalent clinical performance, strength, and intended use. Unlike branded injectables, generic injectable drugs are lower in price and thus are an efficient means to manage healthcare costs. The rising expiration of market exclusivity of various brand-name injectables is contributing to the substantial expansion of the global generic injectables market. Further, the increased initiatives and policies by the government to encourage the use of affordable generic medications are fuelling the Europe generic injectables market demand.The increasing prevalence of chronic diseases like cancer, diabetes, and cardiovascular diseases in the region along with the growing aging population that is more susceptible to developing a health condition is augmenting the demand for cost-effective medications such as generic injectables. Recent data suggests that 1 out of 3 adults in the European Union are reported to be affected by a chronic health problem, with an increasing proportion of chronically ill patients suffering from multi-morbidity. Additionally, older people are more prone to multiple morbidities, with the prevalence reported to be up to 65% and 85% in people aged above 65 and 85 years, respectively. As a result, the demand for healthcare services, including treatments for chronic conditions that often require injectable drugs is projected to rise. Consequently, the rising healthcare needs are estimated to propel the Europe generic injectables market growth.
Strategic partnerships among the key market players are one of the major market trends. In September 2023, Advanz Pharma Corp., a British multinational pharmaceutical company headquartered in London, signed an exclusive agreement with a Spanish biopharmaceutical firm GP Pharm to market and distribute its peptide specialty injectable generic intended for a rare disease in multiple European countries. Such collaborations and distribution agreements to expand market reach and enhance the accessibility of generic injectables are anticipated to bolster the market share in the forecast period.
Europe Generic Injectables Market Segmentation
The report offers a detailed analysis of the market based on the following segments:Market Breakup by Product Type
- Large Molecule Injectables
- Small Molecule Injectables
Market Breakup by Container Type
- Vials
- Premix
- Prefilled Syringes
- Ampoules
- Others
Market Breakup by Application
- Oncology
- Cardiovascular
- CNS
- Infectious Diseases
- Autoimmune Disorders
- Others
Market Breakup by Route of Administration
- Intravenous
- Intramuscular
- Subcutaneous
- Others
Market Breakup by Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Others
Market Breakup by Region
- United Kingdom
- Germany
- France
- Italy
- Others
Leading Players in the Europe Generic Injectables Market
The key features of the market report include patent analysis, grants analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:Pfizer Inc.
The global pharmaceutical giant Pfizer has a strong presence in the market through strategic mergers and acquisitions. The company focuses on high-demand therapeutic areas like oncology and cardiovascular diseases and invests substantially in the development of new and improved generic injectables.Teva Pharmaceutical Industries Ltd.
Headquartered in Israel, this company is a major market player and is involved in rigorous R&D initiatives aimed at expanding its generic product offerings.Baxter
Baxter is another key market player known for its range of generic injectables. The company focuses on ensuring high quality and safety standards in the manufacturing processes of injectables by integration of advanced technologies.Novartis Pharmaceuticals Corporation
Novartis, via its spin-off Sandoz, is heavily involved in the generic injectables sector, especially in developing affordable and effective generic products that address drug shortages in critical care areas.Other players in the market include Fresenius SE & Co. KGaA, Hikma Pharmaceuticals PLC, Dr. Reddy's Laboratories Ltd., Viatris Inc., Biocon, Sanofi, Lupin, and Aurobindo Pharma Limited, among others.
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Pfizer Inc.
- Teva Pharmaceutical Industries Ltd.
- Baxter
- Novartis Pharmaceuticals Corporation
- Fresenius SE & Co. KGaA
- Hikma Pharmaceuticals PLC
- Dr. Reddy’s Laboratories Ltd.
- Viatris Inc.
- Biocon
- Sanofi
- Lupin
- Aurobindo Pharma Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
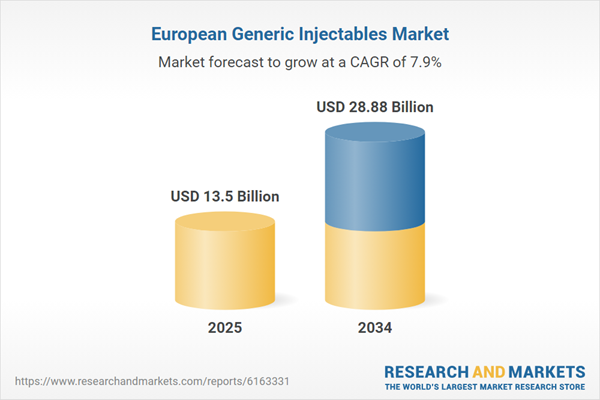
| Estimated Market Value ( USD | $ 13.5 Billion |
| Forecasted Market Value ( USD | $ 28.88 Billion |
| Compound Annual Growth Rate | 7.9% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 12 |