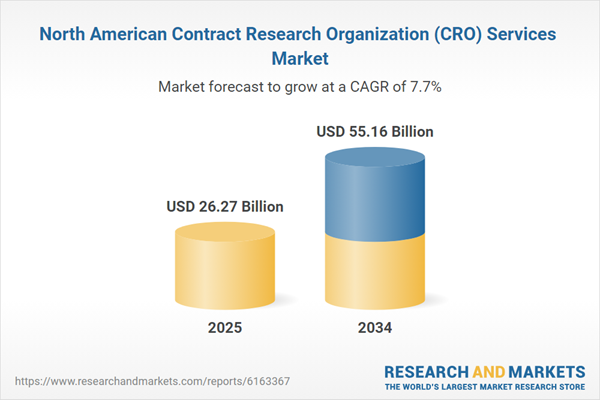
North America Contract Research Organization (CRO) Services Market Overview
Contract research organizations (CROs) offer research-based services to government agencies in the pharmaceutical, biotechnology, and related sectors. They can be either major, full-scale organisations, with an international presence or small, specialised organisations with a narrow focus that provide drug and device research and development (R&D) services, such as drug discovery, preclinical research, and clinical research, among others.The North America contract research organization (CRO) services market is positively impacted by the rising investment in research and development (R&D) by pharmaceutical and biotechnology companies to discover and develop new therapies. Moreover, CROs are increasingly leveraging advanced technologies such as artificial intelligence (AI), machine learning, and big data analytics, in conducting clinical trials, which is stimulating the growth of the global contract research organization (CRO) services market . Other factors influencing the expansion of contract research organizations (CROs) include increased disease monitoring and screening, the requirement for routine diagnostics in healthcare settings, the spread of chronic diseases like diabetes, and the rise in new strains of infectious diseases. These factors are supported by government public health screening policies, the massive increase in patient specimens tested in labs, and the resulting demand for clinical lab testing services.
North America Contract Research Organization (CRO) Services Market Growth Drivers
Growth in Strategic Partnerships to Meet Rising Demand
In September 2023, Parexel International (an American clinical research organization and biopharmaceutical services company) and IT services company Partex signed a deal to combine artificial intelligence-powered solutions with clinical research expertise. The partnership aims to expedite drug discovery and development for biopharmaceutical clients globally and de-risk their assets by predicting the investigational candidates that offer the strongest probability of clinical success. Through this collaborative initiative, Partex intends to enhance customer clinical trial execution by leveraging the Partex-validated AI platform. Such strategic partnerships that prioritize the usage of advanced tools to accelerate the development of safe and effective therapies are poised to fuel market growth in the region.North America Contract Research Organization (CRO) Services Market Trends
The market is witnessing several trends and developments to improve the current scenario in North America. Some of the notable trends are as follows:Expansion of Biopharmaceuticals
One of the major market trends is the increasing development and production of biopharmaceuticals which often need to undergo complex and specialized clinical trials to achieve regulatory approval. Thus, the expansion of biopharmaceutical products propels the demand for CRO services.Increased Focus on Cost-Efficiency
Pharmaceutical and biotechnology companies are increasingly preferring cost-efficient solutions to reduce operational costs and thus are outsourcing R&D and clinical trial activities to CROs to save resources and focus more on their core competencies.Regulatory Requirements
The presence of stringent regulatory requirements for drug approvals bolsters the North America contract research organization (CRO) services market demand. The growing reliance on CROs for regulatory compliance and clinical trial management supports the market expansion.Growing Demand for Patient-Centric Trial Designs
The rising adoption of patient-centric approaches by CROs in clinical trial designs is a major market trend that is improving patient experience and retention. Such trial designs often emphasize incorporating patient feedback and utilizing digital tools to enhance patient engagement.North America Contract Research Organization (CRO) Services Market Segmentation
North America Contract Research Organization (CRO) Services Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Service
- Laboratory Services
- Analytical Testing Services
- Bioanalytical Testing Services
- Preclinical Services
- Research and Development
- Clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Others
Market Breakup by Therapeutic Area
- Oncology
- Cardiovascular
- Infectious Disease
- Neurology
- Gastroenterology and Hepatology
- Respiratory
- Others
Market Breakup by End User
- Pharmaceutical and Biopharmaceutical Companies
- Medical Device Companies
- Research and Academic Institutes
- Others
Market Breakup by Region
- United States
- Canada
North America Contract Research Organization (CRO) Services Market Share
Market Segmentation Based on End Users is Anticipated to Witness Substantial Growth
On the basis of end users, the market can be segmented into pharmaceutical and biopharmaceutical companies, medical device companies, and research and academic institutes, among others. Pharmaceutical and biopharmaceutical companies are predicted to lead the market in terms of revenue throughout the forecast period. The demand for the segment is anticipated to increase as a result of the growing trend of pharmaceutical and biotechnology companies outsourcing end-to-end services, particularly among small and mid-size businesses lacking significant expertise in the preclinical stage of drug development. The prevalence of chronic illnesses like cancer, diabetes, heart disease, and neurological and infectious diseases is also increasing, and pharmaceutical and biopharmaceutical companies are investing more money in creating new treatments, which is anticipated to fuel market expansion.North America Contract Research Organization (CRO) Services Market Analysis by Region
The market segmentation by region includes the United States and Canada. The United States represents the largest share of the market due to the strong presence of leading pharmaceutical and biotechnology companies which drive the demand for CRO services. Further, substantial investments in research and development (R&D) and growth in clinical trials in the region stimulate market growth. Canada is another major market in North America which benefits from robust government support and a favorable environment for clinical research.Leading Players in the North America Contract Research Organization (CRO) Services Market
The key features of the market report comprise funding and investment analysis and strategic initiatives. The major companies in the market are as follows:IQVIA Holdings Inc.
IQVIA Holdings Inc., established in 1982, is a prominent global provider of clinical research services, advanced analytics, and technological solutions to the life sciences industry. Through its analytics, disruptive technologies, in-depth topic understanding, and big data resources, IQVIA forges intelligent linkages across all facets of healthcare. IQVIA is headquartered in North Carolina in the United States.Thermo Fisher Scientific Inc.
Thermo Fisher Scientific Inc., headquartered in the United States, is a provider of scientific equipment, reagents and consumables, and software services. The 1956-founded business provides goods and services that benefit clients all over the world in hospitals and laboratories, on assembly lines, and in the field. It is a driving force in the research, healthcare, industrial, and application markets.Medpace Holdings Inc.
Medpace Holdings Inc., one of the leading key players contributing to the North America contract research organization (CRO) services market growth was established in 1992. It is a full-service, globally active clinical contract research organization (CRO) with a scientifically-driven approach and offers these services to the biotechnology, pharmaceutical, and medical device industries. Through its high-science and disciplined operating approach, which draws on local regulatory and in-depth therapeutic expertise in all important areas, including endocrinology, oncology, cardiology, central nervous system, metabolic disease, and anti-viral and anti-infective therapies, Medpace seeks to accelerate the global development of safe and effective medical therapeutics.Parexel International Corporation
This American provider of biopharmaceutical services has a prominent market presence and is engaged in conducting clinical trials for its pharmaceutical clients in order to accelerate the drug approval process. Parexel International Corporation employs advanced technologies such as artificial intelligence (AI) and big data analytics to improve the efficiency of clinical trials. It often collaborates with pharmaceutical and biotechnology companies to offer end-to-end clinical trial management services.Other key players in the market include Laboratory Corporation of America Holdings, Clinipace, ICON plc, Medpace, Inc., AcceGen Biotechnology, Admescope Ltd., Stelis Biopharma Pvt. Ltd, Almac Group Limited, and Andelyn Biosciences Inc.
Key Questions Answered in the North America Contract Research Organization (CRO) Services Market Report
- What was the North America contract research organization (CRO) services market value in 2024?
- What is the North America contract research organization (CRO) services market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is market segmentation based on services?
- What is the market breakup based on the therapeutic area?
- Who are the major end users in the market?
- What are the major factors aiding the North America contract research organization (CRO) services market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- Which country is expected to experience expedited growth during the forecast period?
- How do the prevalence and incidence of chronic diseases affect the market landscape?
- What are the major North America contract research organization (CRO) services market trends?
- How does the rising investment in R&D activities impact the market size?
- Which service will dominate the market share?
- Which therapeutic area is expected to have a high market value in the coming years?
- Which end user is projected to contribute to the highest market growth?
- Who are the key players involved in the North America contract research organization (CRO) services market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- IQVIA Holdings Inc.
- Thermo Fisher Scientific Inc.
- Medpace Holdings, Inc.
- Parexel International Corporation
- laboratory corporation of America holdings
- Clinipace
- ICON plc
- Medpace, Inc
- AcceGen Biotechnology
- Admescope Ltd.
- Stelis Biopharma Pvt. Ltd
- Almac Group Limited.
- Andelyn Biosciences Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 26.27 Billion |
| Forecasted Market Value ( USD | $ 55.16 Billion |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | North America |
| No. of Companies Mentioned | 13 |