Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
A notable transformation is underway as more institutions adopt minimally invasive and robotic-assisted cardiac surgery. This is not only redefining procedural standards but also compelling procurement departments to prioritize investment in specialized, next-generation instruments that offer enhanced accuracy and integration with digital surgical platforms.
France’s healthcare ecosystem supported by robust public funding, a high surgeon-to-patient ratio, and sustained investment in cardiac care infrastructure is positioning itself as a leader in surgical innovation. The market reflects strong institutional focus on upskilling cardiac surgeons, upgrading operating theaters, and enhancing patient outcomes through the use of smart, high-performance surgical tools.
Key Market Drivers
Rising Prevalence of Cardiovascular Diseases (CVDs)
The most critical drivers of growth in the France Cardiac Surgery Instruments Market is the rising prevalence of cardiovascular diseases (CVDs). As the burden of heart-related conditions escalates across the country, there is a parallel surge in demand for surgical interventions, thereby fueling the need for advanced and precise cardiac surgery instruments. In 2021, cardiovascular diseases (CVD) accounted for 152,728 deaths in France, highlighting their continued impact on national health outcomes. Despite this high absolute number, France ranks within the lowest 20% of countries globally for age-standardized CVD mortality rates indicating comparatively better outcomes in managing cardiovascular risk factors and access to treatment.CVDs, including coronary artery disease (CAD), heart failure, arrhythmias, and valvular disorders, have become leading causes of mortality and morbidity in France. Lifestyle-related risk factors such as sedentary habits, poor nutrition, obesity, smoking, and hypertension. Rising incidence of comorbidities like type 2 diabetes and dyslipidemia. In France, the prevalence of clinically diagnosed type 2 diabetes stands at approximately 5% of the population, though this figure varies across different demographic groups and regional studies. As these conditions often lead to acute or chronic cardiac complications requiring surgical treatment, the demand for high-quality surgical instruments is expected to rise significantly.
Many CVDs progress to stages where interventional or surgical procedures become the most viable treatment options. This includes Coronary Artery Bypass Grafting (CABG) for severe coronary artery blockages, Valve replacement or repair surgeries for patients with stenosis or regurgitation, Pacemaker or defibrillator implantation in cases of life-threatening arrhythmias, Congenital defect correction surgeries in pediatric and adult populations. Each of these procedures requires a diverse range of surgical tools such as vascular forceps, scissors, needle holders, retractors, clamps, and sternum spreaders. As procedural volumes increase, hospitals and surgical centers are compelled to invest more in high-performance, reliable instruments.
The high burden of CVDs is pushing public and private hospitals in France to expand their cardiac surgery capabilities. This includes Establishing dedicated cardiac surgery departments in regional and tertiary-care hospitals, Increasing the number of operating theaters and specialized cardiac ICU beds, Training more cardiovascular surgeons and supporting surgical teams. This expansion is directly tied to greater procurement of surgical instruments, as hospitals aim to keep up with rising procedural demand and ensure surgical precision and safety.
Key Market Challenges
Stringent Regulatory Framework and Lengthy Approval Processes
France, as part of the European Union, adheres to strict medical device regulations under the EU Medical Device Regulation (MDR 2017/745). This regulatory environment, while essential for ensuring patient safety and product efficacy, creates several operational challenges for manufacturers of cardiac surgery instruments.Lengthy and complex approval timelines for new surgical instruments delay product launches and limit the speed of innovation. Compliance with rigorous documentation, clinical evaluation, and post-market surveillance requirements increases time-to-market and inflates operational costs. Small and mid-sized companies, in particular, face difficulties navigating these regulatory hurdles, leading to reduced competition and slower market expansion. As a result, innovation is often stifled, and access to the latest high-performance instruments is delayed for hospitals and surgical centers in France.
Key Market Trends
Surge in Minimally Invasive and Robotic-Assisted Cardiac Surgeries
One of the most transformative trends in the cardiac surgery landscape is the growing shift toward minimally invasive cardiac surgery (MICS) and robotic-assisted procedures. French hospitals, especially university-affiliated and private centers in regions like Northern and Western France, are increasingly adopting advanced surgical systems such as the da Vinci robotic platform.These procedures require high-precision, micro-sized surgical instruments that can navigate tight anatomical spaces with minimal tissue trauma. Robotic-assisted surgery necessitates specialized instrument sets compatible with robotic arms and endoscopic visualization. As these technologies reduce recovery times, hospital stays, and postoperative complications, their adoption is accelerating thereby expanding the market for next-generation surgical tools.
This trend is expected to significantly influence procurement patterns, encouraging suppliers to focus on innovation, miniaturization, and ergonomic instrument design.
Key Market Players
- Teleflex Medical SAS
- Medline International France SAS
- B. Braun SE
- KLS Martin France SARL
- Wexler Surgical
Report Scope:
In this report, the France Cardiac Surgery Instruments Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:France Cardiac Surgery Instruments Market, By Product:
- Forceps
- Clamps
- Scalpels
- Scissors
- Needle Holders
- Others
France Cardiac Surgery Instruments Market, By Application:
- Coronary Artery Bypass Grafting
- Heart Valve Surgery
- Pediatric Surgery
- Heart Transplant
- Others
France Cardiac Surgery Instruments Market, By End User:
- Hospitals
- Ambulatory Surgical Centers
France Cardiac Surgery Instruments Market, By Region:
- Northern France
- Western France
- Southern France
- Eastern France
- Central France
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the France Cardiac Surgery Instruments Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Teleflex Medical SAS
- Medline International France SAS
- B. Braun SE
- KLS Martin France SARL
- Wexler Surgical
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 85 |
| Published | August 2025 |
| Forecast Period | 2024 - 2030 |
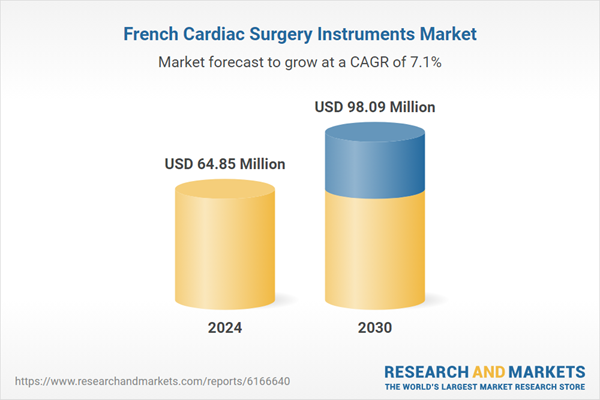
| Estimated Market Value ( USD | $ 64.85 Million |
| Forecasted Market Value ( USD | $ 98.09 Million |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | France |
| No. of Companies Mentioned | 5 |