Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key trends shaping the market include the integration of artificial intelligence in biotechnology, which enhances the development of microbiome-based therapeutics. Contract Development and Manufacturing Organizations (CDMOs) are playing a crucial role in scaling these therapies, from strain development to commercial manufacturing. This growth is supported by rising chronic disease prevalence, advancements in microbiome research, increasing demand for personalized medicine, and growing investments in R&D.
Despite the promising outlook, the market faces challenges such as prolonged regulatory approval processes and complex legal frameworks. The lack of standardized guidelines for production, quality control, and safety assessment complicates the assurance of product consistency and reliability. These challenges hinder the progress of live biotherapeutic products, necessitating ongoing innovation and technological advancements to overcome these barriers.
Key Market Drivers
Increasing Prevalence of Chronic Diseases
The increasing prevalence of chronic diseases is a primary market driver for the United States Live Biotherapeutics market. Conditions such as inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), diabetes, obesity, and certain autoimmune and metabolic disorders are reaching epidemic proportions, creating a substantial and expanding patient population.This growth is quantifiable; data from the Centers for Disease Control and Prevention (CDC) indicates that as of 2023, six in ten adults in the U.S. live with a chronic disease, and four in ten have two or more. The National Health Interview Survey (NHIS) data reveals that in 2022, 11.3% of U.S. adults had been diagnosed with IBD, representing millions of potential patients. This surge creates a population actively seeking effective, long-term management solutions beyond conventional pharmaceuticals, which often provide symptomatic relief with significant side effects or diminishing efficacy.
The limitations of traditional therapies, including antibiotics and immunosuppressants, have exposed a critical unmet medical need for novel treatment modalities that address the underlying pathophysiology of these complex conditions. Scientific research has fundamentally established a strong link between gut microbiome dysbiosis and the pathogenesis of numerous chronic illnesses. This understanding positions Live Biotherapeutics, defined biological products containing live organisms, as a targeted therapeutic strategy.
For a patient population weary of treatments that merely manage symptoms, the potential of these products to restore natural gut function and modify the disease course offers a compelling value proposition. This driver is amplified by a growing body of clinical evidence demonstrating the efficacy of these microbial interventions, encouraging greater investment from pharmaceutical companies and fostering acceptance within the medical community. The escalating healthcare burden, underscored by CDC reports that chronic diseases are the leading cause of death and disability and account for the majority of the nation's USD 4.5 trillion in annual health care costs, further incentivizes the development of these innovative, potentially curative therapies.
Key Market Challenges
Regulatory Uncertainty and Evolving Guidelines
Regulatory uncertainty remains one of the major challenges for the United States Live Biotherapeutics Market. As live biotherapeutic products (LBPs) involve novel and diverse microbial strains, regulators are faced with challenges in establishing clear guidelines. The evolving nature of these regulations can create significant obstacles for manufacturers, as compliance requirements may change or remain unclear, leading to delays in product approvals. Regulatory bodies such as the FDA, EMA, and other global authorities are still developing frameworks to address the unique characteristics of live biotherapeutics, which often do not fit within traditional pharmaceutical regulations.These evolving guidelines can result in extended approval timelines and increased costs for companies attempting to bring innovative therapies to market. Additionally, the global nature of the market requires adherence to multiple regulatory standards, complicating the approval process for manufacturers who wish to introduce their products in various regions. The lack of harmonization between different regulatory bodies adds a layer of complexity for businesses, leading to higher operational costs and uncertain market entry strategies. Companies must continually monitor regulatory changes and adapt their strategies accordingly, which can be resource-intensive and slow down market growth.
Key Market Trends
Rising Interest in Microbiome-Based Therapeutics
The growing interest in microbiome-based therapeutics is significantly impacting the United States Live Biotherapeutics Market. Researchers and pharmaceutical companies have increasingly turned their attention to the human microbiome for its therapeutic potential, recognizing its role in numerous physiological processes, including immune response, metabolism, and disease prevention. As the understanding of the microbiome’s influence on health deepens, it is becoming a focal point for the development of innovative treatments for a wide range of diseases, including autoimmune disorders, gastrointestinal diseases, and even cancer. This shift in focus is driving the demand for live biotherapeutic products that are designed to modulate or restore the balance of microbiota.The increasing number of microbiome-related clinical trials further highlights this trend. Regulatory bodies, such as the FDA and EMA, are establishing frameworks for approving microbiome-based therapies, making it more feasible for companies to bring their microbiome-related products to market. As the field continues to evolve, there is growing interest in the development of personalized microbiome therapies, tailored to individuals’ unique microbiota profiles. This level of personalization is enhancing the market’s potential by opening avenues for more effective treatments. The rising interest in microbiome-based therapeutics is also being bolstered by advancements in sequencing technology, which allows for more precise identification of beneficial microorganisms that can be used for therapeutic purposes. This trend is expected to continue expanding the market for live biotherapeutics and microbial contract development and manufacturing organizations (CDMOs).
Key Market Players
- Arrant Bio
- 4D Pharma
- Cerbios
- Biose Industrie
- Assembly Biosciences, Inc.
- Wacker Chemie AG
- Quay Pharmaceuticals
- NIZO
- Lonza
- Inpac Probiotics
Report Scope:
In this report, the United States Live Biotherapeutics Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:United States Live Biotherapeutics Market, By Application:
- C.difficle
- Crohns Disease
- IBS
- Diabetes
- Others
United States Live Biotherapeutics Market, By Product:
- APIs
- FDFs
United States Live Biotherapeutics Market, By Type of Formulation:
- Solid Formulations
- Oral Liquids
- Injectables
- Others
United States Live Biotherapeutics Market, By Scale of Operation:
- Preclinical Scale Operations
- Clinical Scale Operations
- Commercial Scale Operations
United States Live Biotherapeutics Market, By Region:
- North-East
- Mid-West
- West
- South
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the United States Live Biotherapeutics Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Arrant Bio
- 4D Pharma
- Cerbios
- Biose Industrie
- Assembly Biosciences, Inc.
- Wacker Chemie AG
- Quay Pharmaceuticals
- NIZO
- Lonza
- Inpac Probiotics
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 84 |
| Published | August 2025 |
| Forecast Period | 2024 - 2030 |
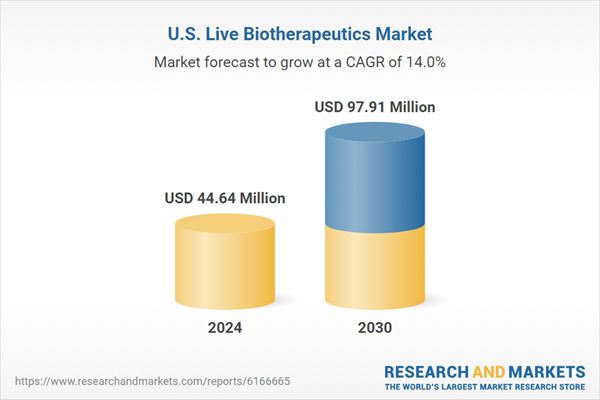
| Estimated Market Value ( USD | $ 44.64 Million |
| Forecasted Market Value ( USD | $ 97.91 Million |
| Compound Annual Growth Rate | 13.9% |
| Regions Covered | United States |
| No. of Companies Mentioned | 10 |