Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Increasing awareness among healthcare providers and patients regarding the benefits of biologic therapies, coupled with advancements in clinical research, is fueling adoption. Pharmaceutical companies are focusing on expanding their biologic portfolios, investing in research and development to optimize efficacy, minimize side effects, and develop patient-friendly administration methods. The growing emphasis on personalized medicine, which tailors treatments to individual patient profiles, is also boosting demand for innovative biologics like Rozanolixizumab, as it aligns with the trend of precision therapy for complex autoimmune conditions.
Key Market Drivers
Rising Prevalence of Autoimmune Disorders
The increasing prevalence of autoimmune disorders significantly drives the Global Rozanolixizumab (Rystiggo) Market, highlighting an urgent need for targeted therapeutic interventions. Generalized myasthenia gravis (gMG), a chronic autoimmune neuromuscular disorder characterized by fluctuating muscle weakness, affects approximately 82,715 adults in the United States as of 2021, equating to a prevalence of 37.0 per 100,000 persons. This condition predominantly impacts individuals under 50 years of age, with a higher incidence observed in females. Rozanolixizumab, an FcRn inhibitor, offers a novel treatment approach by selectively reducing pathogenic IgG antibodies, addressing the underlying pathophysiology of gMG.Immune thrombocytopenia (ITP), another autoimmune disorder characterized by a low platelet count leading to increased bleeding risk, also contributes to the market's growth. The incidence of ITP in adults ranges from 1 to 5 per 100,000 individuals, with a higher prevalence due to the chronic nature of the disorder. These statistics underscore the increasing burden of autoimmune diseases globally, driving the demand for effective therapies like Rozanolixizumab. As the prevalence of such conditions rises, healthcare providers are seeking targeted treatments that offer improved efficacy and safety profiles, thereby propelling the adoption of Rozanolixizumab in clinical practice.
Rozanolixizumab's mechanism of action, which involves the inhibition of the neonatal Fc receptor (FcRn), allows for the selective reduction of pathogenic IgG antibodies without broadly suppressing the immune system. This targeted approach offers an alternative to conventional immunosuppressive therapies, which are often associated with broader immune suppression and significant side effects.
The growing number of patients diagnosed with autoimmune conditions has led healthcare providers to seek therapies that offer higher efficacy, safety, and patient compliance, positioning Rozanolixizumab as a preferred option. Expansion of healthcare facilities specializing in chronic and complex autoimmune diseases, alongside increased access to infusion centers and specialized clinics, further supports adoption. Patient advocacy and educational initiatives are encouraging early intervention and treatment adherence, reinforcing the market demand. As autoimmune disorders continue to rise globally, the need for innovative biologics like Rozanolixizumab is expected to grow steadily, driving market expansion and creating opportunities for pharmaceutical companies to develop accessible, effective therapies for these challenging conditions.
Key Market Challenges
High Treatment Costs
A significant challenge for the Global Rozanolixizumab (Rystiggo) Market is the high cost of treatment, which can limit patient access and adoption across both developed and emerging markets. As a novel monoclonal antibody therapy, Rozanolixizumab involves complex biologic manufacturing processes, stringent quality control measures, and extensive clinical research, all of which contribute to elevated pricing. For patients, especially those without comprehensive insurance coverage or in regions with limited healthcare funding, the financial burden can be a major barrier to initiating or continuing therapy. High treatment costs may also affect healthcare providers’ willingness to prescribe Rozanolixizumab, particularly when more affordable alternative therapies, such as conventional immunosuppressants or competing biologics, are available.The pricing challenge is amplified by the chronic nature of autoimmune disorders, which often require long-term or repeated dosing to achieve sustained therapeutic outcomes. Patients may face recurring expenses, including monitoring, follow-up visits, and supportive care, which further increases the total cost of treatment. Payers and insurers may impose restrictions on coverage or require prior authorizations, delaying access and creating administrative hurdles. High costs can also influence market penetration in emerging economies, where per capita healthcare spending is limited and patients may prioritize essential medications over specialty biologics.
Companies are attempting to address this challenge through patient assistance programs, financial support initiatives, and flexible pricing strategies. Programs that subsidize out-of-pocket costs, offer co-pay assistance, or provide treatment access through specialty pharmacies can help mitigate financial barriers and encourage adoption. Despite these efforts, the high cost of Rozanolixizumab remains a critical concern for healthcare providers, payers, and patients, representing a key challenge that the market must navigate to achieve broader acceptance and sustainable growth.
Key Market Trends
Shift Toward Targeted Biologic Therapies
A prominent trend driving the Global Rozanolixizumab (Rystiggo) Market is the growing shift toward targeted biologic therapies, reflecting a broader evolution in the management of autoimmune and immune-mediated disorders. Traditional treatment options, such as corticosteroids and immunosuppressants, often affect the immune system broadly, leading to systemic side effects and variable efficacy. Rozanolixizumab, a monoclonal antibody that selectively inhibits the neonatal Fc receptor (FcRn), offers a more precise approach by reducing pathogenic IgG antibodies without compromising overall immune function. This targeted mechanism allows healthcare providers to tailor treatments to the underlying pathophysiology of specific autoimmune diseases, improving therapeutic outcomes and reducing adverse events.The shift toward biologics aligns with increasing emphasis on personalized medicine, where therapies are selected based on patient-specific profiles, including antibody levels, disease severity, and previous treatment responses. Clinicians are adopting Rozanolixizumab as part of individualized treatment plans to optimize efficacy and safety, particularly for patients with generalized myasthenia gravis and other IgG-mediated disorders. The development of patient-friendly delivery methods, such as subcutaneous formulations, has made targeted biologics more accessible and convenient, encouraging broader adoption among both healthcare providers and patients.
Rising awareness among healthcare professionals about the benefits of FcRn inhibition, coupled with strong clinical evidence supporting Rozanolixizumab’s safety and effectiveness, is reinforcing confidence in biologic therapies. Investments in research and development are further expanding the pipeline of targeted therapies, enabling treatment of a wider range of autoimmune conditions. As the healthcare industry continues to prioritize precision, efficacy, and improved quality of life for patients with chronic immune disorders, the shift toward targeted biologic therapies is expected to remain a defining trend in the Global Rozanolixizumab Market.
Key Market Player
- UCB Pharma S.A.
Report Scope:
In this report, the Global Rozanolixizumab (Rystiggo) Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Rozanolixizumab (Rystiggo) Market, By Indication:
- Generalized Myasthenia Gravis (gMG)
- Others
Rozanolixizumab (Rystiggo) Market, By Distribution Channel:
- Hospital & Specialty Pharmacies
- Retail & E-Commerce
Rozanolixizumab (Rystiggo) Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Rozanolixizumab (Rystiggo) Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- UCB Pharma S.A.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | August 2025 |
| Forecast Period | 2024 - 2030 |
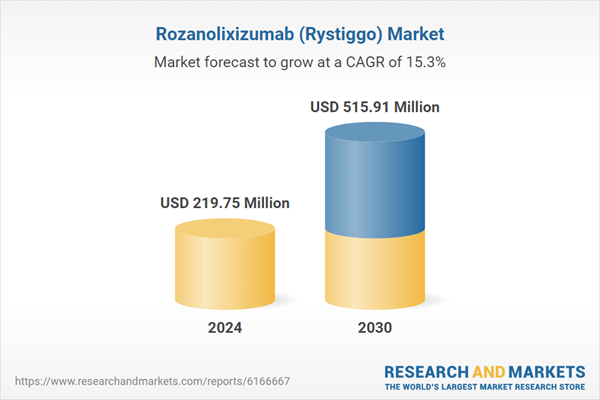
| Estimated Market Value ( USD | $ 219.75 Million |
| Forecasted Market Value ( USD | $ 515.91 Million |
| Compound Annual Growth Rate | 15.2% |
| Regions Covered | Global |