Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
These technologies provide high-resolution, non-invasive imaging capabilities that facilitate accurate diagnosis and effective treatment planning. The integration of artificial intelligence (AI) and machine learning algorithms in imaging devices is further enhancing diagnostic accuracy, enabling clinicians to identify malignant and pre-malignant lesions more efficiently. The demand for non-invasive and patient-friendly diagnostic solutions is driving healthcare providers to invest in state-of-the-art equipment to meet the growing clinical requirements while improving patient outcomes and satisfaction.
Technological advancements and innovation are major factors fueling the market’s expansion. Imaging devices are increasingly equipped with AI-assisted analysis, high-definition imaging, and 3D visualization capabilities, which aid in precise lesion mapping, monitoring of treatment response, and early detection of subtle skin abnormalities. The rising number of dermatology clinics, outpatient centers, and teledermatology services is boosting the utilization of diagnostic imaging devices, as clinicians seek faster and more accurate diagnostic solutions to manage growing patient loads. Furthermore, investments by hospitals and specialized dermatology centers in upgrading diagnostic infrastructure to incorporate cutting-edge devices have accelerated market penetration. Trends such as portable imaging devices, integration with electronic health records, and real-time remote diagnostics are redefining patient management and enabling better clinical decision-making.
Despite strong growth, the market faces several challenges. High costs of advanced imaging systems can restrict adoption among smaller clinics and outpatient centers with limited budgets. Limited awareness among some healthcare professionals regarding the latest diagnostic technologies and the need for specialized training to operate sophisticated devices may slow adoption rates.
Regulatory requirements and approval processes for new devices can delay market entry and affect product commercialization timelines. Competition from alternative diagnostic methods, including visual inspection and biopsy-based assessments, also poses challenges for wider market acceptance. Ensuring data security and privacy in AI-enabled and cloud-integrated imaging platforms remains a critical concern for healthcare providers. Addressing these barriers while focusing on technological advancements and clinician training will be crucial for sustaining the growth trajectory of the United States Diagnostic Dermatology Imaging Devices Market in the coming years.
Key Market Drivers
Rising Incidence of Skin Disorders and Skin Cancer
The rising incidence of skin disorders and skin cancer is significantly accelerating the demand for diagnostic dermatology imaging devices worldwide. In the United States alone, skin cancer represents the most common type of cancer, with approximately 5.4 million cases diagnosed annually. Data from the CDC shows that over 6.1 million adults are treated annually for basal and squamous cell carcinomas, incurring nearly USD 8.9 billion a year in medical costs.New cases of melanoma which, while less common, are more aggressive are also on the rise. In 2024, approximately 200,340 melanoma cases (comprising 99,700 in situ and 100,640 invasive) are expected.
Conventional examination methods, including visual and manual assessments, often miss subtle lesion changes and lack depth resolution. Imaging devices such as dermatoscopes, reflectance confocal microscopes, and optical coherence tomography provide non-invasive, real-time visualization at the cellular level, significantly enhancing diagnostic accuracy and accelerating lesion detection. These tools reduce reliance on biopsies and enable earlier intervention, which is linked to improved survival rates early-stage melanoma shows a five-year relative survival rate above 94%.
Growing public awareness of the importance of early skin examinations is supporting wider adoption of diagnostic imaging. The CDC’s Melanoma Dashboard and public health initiatives are advocating regular screenings, especially among high-risk groups, which leads to increased device utilization in both primary and specialist settings. Portable, AI-enhanced imaging systems are now more feasible and cost-effective, bringing advanced diagnostic capability to clinics, remote health centers, and teledermatology networks.
With skin cancer and other skin conditions continuing to rise and represent significant healthcare burdens both clinically and economically, clinicians and health systems are increasingly investing in imaging technology. The ability to detect lesions with precision, track therapeutic progress, and reduce unnecessary invasive procedures positions diagnostic imaging tools as essential components in the evolving paradigm of dermatological care.
Key Market Challenges
High Cost of Advanced Imaging Systems
The high cost of advanced imaging systems presents a significant challenge to the widespread adoption of diagnostic dermatology devices across various healthcare settings. These imaging systems, which include high-resolution digital dermatoscopes, reflectance confocal microscopes, and optical coherence tomography scanners, require substantial upfront investment. Many healthcare providers, particularly those in small clinics or resource-limited regions, find it financially burdensome to acquire and maintain such equipment. The expense is not limited to initial purchase but extends to regular software updates, technical maintenance, and training of medical personnel to use the equipment effectively. This financial barrier restricts accessibility and results in limited deployment of advanced imaging tools in rural or underfunded medical facilities, reducing early detection capabilities in underserved populations.The pricing of cutting-edge dermatology imaging devices is also influenced by the incorporation of AI-driven algorithms, 3D imaging capabilities, and cloud-based data storage systems. These features, while enhancing diagnostic accuracy and operational efficiency, further increase the total cost of ownership. Health systems that operate on tight budgets or under public healthcare models often prioritize more pressing infrastructure or clinical needs, leaving dermatology imaging investments deprioritized.
In developing nations, where reimbursement mechanisms for dermatology diagnostics are either absent or inadequate, the return on investment for such costly devices becomes even less attractive. This cost-related constraint slows down market penetration and limits the commercial viability of newer technologies. Without cost-effective solutions or innovative pricing models such as leasing or pay-per-use systems, the market growth potential remains constrained despite increasing demand for dermatological diagnostics. The financial inaccessibility of these systems continues to be a critical barrier that manufacturers and healthcare policymakers must address to ensure equitable distribution and utilization of advanced diagnostic dermatology imaging technologies.
Key Market Trends
Growth of Teledermatology and Remote Diagnostics
The United States Diagnostic Dermatology Imaging Devices Market is experiencing a significant shift due to the rising adoption of teledermatology and remote diagnostic technologies. The growing need for accessible dermatological care, particularly in rural and underserved regions, has led to the development and deployment of portable, cloud-integrated imaging devices that allow real-time skin assessments and consultations.These solutions enable patients to capture and transmit high-resolution images of skin conditions through secure platforms, where dermatologists can evaluate and provide accurate diagnoses remotely. This model not only enhances patient convenience but also optimizes the workflow of dermatology professionals by reducing in-person consultation time and enabling quicker triage of cases. The trend has been further supported by the increasing penetration of smartphones and internet connectivity, creating an ecosystem where mobile imaging solutions are becoming viable tools for early skin disease detection.
Artificial intelligence and machine learning algorithms are playing a critical role in this trend, as they assist in image interpretation, pattern recognition, and diagnostic suggestions, significantly reducing the chances of human error. Startups and established medical device firms are investing heavily in the integration of AI-powered platforms with dermatology imaging tools to facilitate faster clinical decision-making. Health systems and payers are also showing growing interest in remote diagnostics due to its cost-effectiveness and ability to reduce the burden on outpatient facilities.
With regulatory bodies beginning to recognize and support teledermatology practices through new reimbursement codes and policy frameworks, adoption is expected to rise sharply in the coming years. This shift is fostering a decentralized model of dermatological care, where imaging devices are not confined to hospitals or clinics but are accessible through home care, primary care, and mobile health units. The expansion of teledermatology is not only broadening the market reach but also redefining the standard of skin health management worldwide.
Key Market Players
- Canfield Scientific
- Caliber Imaging & Diagnostics
- Michelson Diagnostics (MDL)
- SciBase AB
- DermaSensor Inc.
- Damae Medical
- Apollo Medical Optics
- Enspectra Health, Inc.
- Dermavision Solutions
- Dermus Ltd.
Report Scope:
In this report, the United States Diagnostic Dermatology Imaging Devices Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:United States Diagnostic Dermatology Imaging Devices Market, By Modality:
- Advanced Diagnostic Imaging Devices
- Surface Visualization Devices
- Biopsy Devices
- Molecular Diagnostic Devices & Platforms
United States Diagnostic Dermatology Imaging Devices Market, By Application:
- Skin Cancer
- Inflammatory & Autoimmune Skin Diseases
- Infectious Skin Conditions
- Wound & Ulcer Assessment
United States Diagnostic Dermatology Imaging Devices Market, By End User:
- Hospitals
- Dermatology Clinics/Centers
- Cancer Centers/Oncology Clinics
- Others
United States Diagnostic Dermatology Imaging Devices Market, By Region:
- North-East
- Mid-West
- West
- South
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the United States Diagnostic Dermatology Imaging Devices Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Canfield Scientific
- Caliber Imaging & Diagnostics
- Michelson Diagnostics (MDL)
- SciBase AB
- DermaSensor Inc.
- Damae Medical
- Apollo Medical Optics
- Enspectra Health, Inc.
- Dermavision Solutions
- Dermus Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 84 |
| Published | August 2025 |
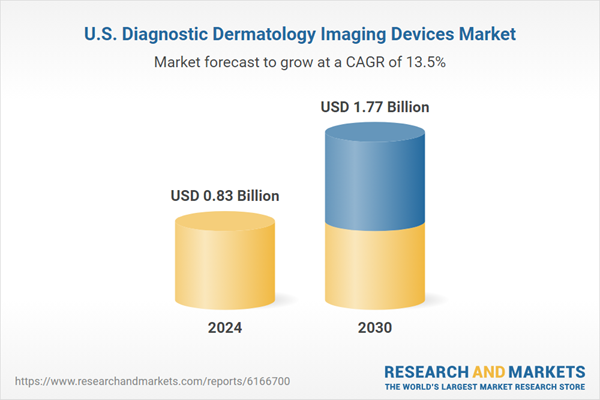
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 0.83 Billion |
| Forecasted Market Value ( USD | $ 1.77 Billion |
| Compound Annual Growth Rate | 13.5% |
| Regions Covered | United States |
| No. of Companies Mentioned | 10 |