The U.S. HPV testing industry is undergoing rapid transformation, fueled by updated clinical guidelines, landmark regulatory approvals, and a shift toward patient-centered screening models. In July 2020, the American Cancer Society (ACS) updated its recommendations, making primary HPV testing every five years the preferred method for individuals aged 25-65, citing superior accuracy, longer screening intervals, and reduced unnecessary interventions compared with Pap smears. This move reflects growing evidence that HPV testing more reliably detects high-grade precancerous lesions while avoiding the overdiagnosis associated with cytology.
In December 2024, the U.S. Preventive Services Task Force (USPSTF) released draft guidelines recommending HPV testing every five years for women aged 30-65, with Pap testing or HPV/Pap co-testing as acceptable alternatives. Critically, the USPSTF included self-collected HPV samples taken in healthcare settings for the first time, acknowledging studies showing comparable accuracy to clinician-collected specimens and significantly higher uptake among historically underscreened groups.
A major regulatory milestone followed in May 2024, when the FDA approved HPV self-collection for cervical cancer screening in clinical environments. ACS CEO stated, “Self-collection can expand access to screening and reduce barriers, giving more people the opportunity to detect, treat, and ultimately survive cancer.” On the same day, Roche secured FDA approval for its cobas HPV self-collection solution, enabling patients to privately collect vaginal samples for laboratory testing. Roche Diagnostics CEO emphasized, “Our HPV self-collection solution helps support the goal of eliminating cervical cancer by 2030 by reducing barriers and providing access to HPV screening.”
With 13,000 new cervical cancer diagnoses and 4,000 related deaths annually in the U.S.-over half in underscreened populations-these combined initiatives are poised to close critical screening gaps. Public-private collaborations, such as the National Cancer Institute’s Cervical Cancer “Last Mile” Initiative, are aligning with WHO’s 2030 elimination strategy to expand reach, modernize screening pathways, and ensure equity in access. As adoption of self-collection and guideline-driven HPV testing accelerates, the U.S. HPV testing market is expected to see robust growth, driven by increased demand for high-sensitivity molecular assays, broader participation, and expanded reimbursement coverage.
U.S. Human Papillomavirus Testing Market Report Segmentation
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. The analyst has segmented the U.S. HPV testing market report on the basis of application, product, technology, and end use:Application Outlook (Revenue, USD Million, 2021-2033)
- Cervical Cancer Screening
- Vaginal Cancer Screening
Product Outlook (Revenue, USD Million, 2021-2033)
- Instruments
- Consumables
- Services
Technology Outlook (Revenue, USD Million, 2021-2033)
- PCR
- Immunodiagnostics
- Others
End Use Outlook (Revenue, USD Million, 2021-2033)
- Hospitals & Clinics
- Laboratories
- Others
Why should you buy this report?
- Comprehensive Market Analysis: Gain detailed insights into the industry across major regions and segments.
- Competitive Landscape: Explore the market presence of key players.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segmental and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listings for you to stay ahead of the curve
- COVID-19's impact and how to sustain in these fast-evolving markets
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The major companies profiled in this U.S. Human Papillomavirus Testing market report include:- Abbott
- Biomedical Diagnostics
- bioMerieux
- Bio-Rad Laboratories, Inc.
- Fujirebio
- Oncolab
- Hologic, Inc.
- Qiagen
- F. Hoffmann-La Roche Ltd
- Siemens Healthineers AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 150 |
| Published | August 2025 |
| Forecast Period | 2024 - 2033 |
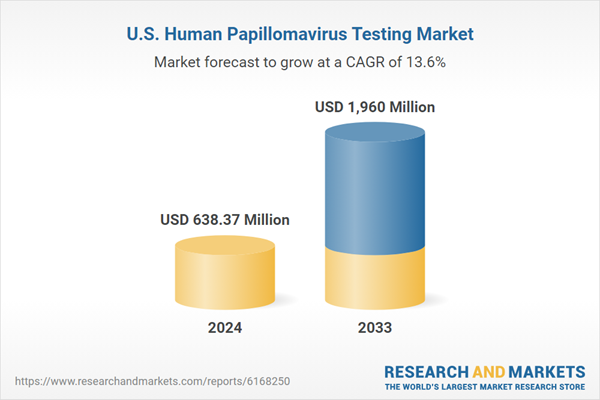
| Estimated Market Value ( USD | $ 638.37 Million |
| Forecasted Market Value ( USD | $ 1960 Million |
| Compound Annual Growth Rate | 13.5% |
| Regions Covered | United States |
| No. of Companies Mentioned | 11 |