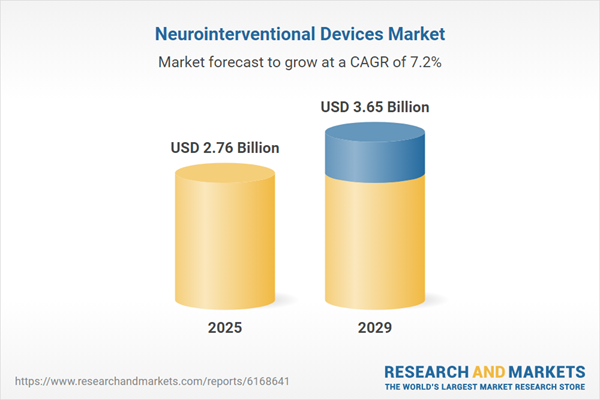
The neurointerventional devices market size is expected to see strong growth in the next few years. It will grow to $3.65 billion in 2029 at a compound annual growth rate (CAGR) of 7.2%. The growth projected for the forecast period can be attributed to the increasing preference for image-guided surgeries, rising investment in neurovascular research, growing approvals of novel neurointerventional products, expanding partnerships and acquisitions among device manufacturers, and heightened demand for personalized treatment approaches. Key trends during this period include advancements in robotic-assisted neurointerventions, innovations in catheter and stent design, enhanced integration of artificial intelligence in diagnostic imaging, incorporation of augmented reality in surgical planning, and developments in embolization and thrombectomy techniques.
The increasing incidence of neurological disorders is expected to drive growth in the neurointerventional devices market going forward. Neurological disorders encompass any conditions affecting the brain, spinal cord, or nerves that disrupt normal functioning. This rise is largely attributed to an aging global population, as advancing age heightens the risk of neurodegenerative diseases due to natural declines in brain function and cellular repair. Neurointerventional devices assist in treating neurological disorders by enabling minimally invasive procedures to diagnose and manage conditions like brain aneurysms, strokes, and vascular malformations, which reduces risks, shortens recovery time, and improves patient outcomes. For example, according to a report by the Alzheimer's Association, a US-based nonprofit, 6.5 million Americans aged 65 and older were diagnosed with Alzheimer's dementia in 2022, rising to 6.9 million in 2024. Therefore, the growing incidence of neurological disorders is driving the neurointerventional devices market.
Key players in the neurointerventional devices market are focusing on technological innovations such as AI-assisted navigation systems to improve procedural accuracy, reduce intervention time, and enhance patient outcomes in complex neurovascular treatments. AI-assisted navigation systems use artificial intelligence algorithms to guide neurointerventional procedures in real time by analyzing medical imaging data. These systems provide enhanced visualization, automate path planning, and assist in accurately navigating devices through intricate brain vasculature, thereby increasing precision, shortening procedure durations, and minimizing risks. For instance, in October 2022, Medtronic plc, an Ireland-based healthcare technology company, launched the Medtronic Neurovascular Co Lab Platform to accelerate stroke treatment innovation. This platform encourages collaboration among startups, physicians, and institutions by offering a continuous virtual portal for submitting ideas, which are reviewed and supported through Medtronic’s expertise, funding, and network to rapidly develop promising neurovascular solutions and improve patient care.
In September 2024, Boston Scientific, a US-based medical technology company, acquired Silk Road Medical for approximately $1.18 billion. Through this acquisition, Boston Scientific aims to broaden its vascular surgery portfolio, strengthen its stroke prevention offerings, and accelerate its mission to transform treatment approaches for vascular disease. Silk Road Medical is a US-based company specializing in medical devices such as transcarotid artery revascularization systems, including the ENROUTE transcarotid neuroprotection and stent systems.
Major players in the neurointerventional devices market are Johnson & Johnson, Abbott Laboratories, Medtronic plc, Stryker Corporation, Boston Scientific Corporation, Terumo Corporation, Kaneka Corporation, Cook Medical LLC, Integra LifeSciences Holdings Corporation, Merit Medical Systems Inc., MicroPort Scientific Corporation, Penumbra Inc., Asahi Intecc Co. Ltd., MicroVention Inc., Phenox GmbH, Balton Sp. z o.o., Zylox-Tonbridge Medical Technology Co. Ltd., Balt Group SAS, Veiva Scientific Co. Ltd., and InspireMD Inc.
North America was the largest region in the neurointerventional market in 2024. The regions covered in neurointerventional devices report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the neurointerventional devices market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The neurointerventional devices market consists of sales of catheters, stents, embolic coils, thrombectomy devices, and guidewires. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The fast surge in U.S. tariffs and the trade tensions that followed in spring 2025 are heavily affecting the medical equipment sector, particularly for imported imaging machine components, surgical-grade stainless steel, and plastic disposables. Hospitals and clinics resist price hikes, pressuring manufacturers’ margins. Regulatory hurdles compound the problem, as tariff-related supplier changes often require re-certification of devices, delaying time-to-market. Companies are mitigating risks by dual-sourcing critical parts, expanding domestic production of commoditized items, and accelerating R&D in cost-efficient materials.
The neurointerventional devices market research report is one of a series of new reports that provides neurointerventional devices market statistics, including the neurointerventional devices industry global market size, regional shares, competitors with the neurointerventional devices market share, detailed neurointerventional devices market segments, market trends, and opportunities, and any further data you may need to thrive in the neurointerventional devices industry. This neurointerventional devices market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
Neurointerventional devices are specialized medical instruments designed for minimally invasive procedures aimed at diagnosing and treating disorders of the blood vessels in the brain, spine, head, and neck. Their main function is to restore or enhance blood flow, remove clots, repair aneurysms, or address vascular malformations without requiring open surgery.
The primary types of neurointerventional devices include embolic coils, carotid stents, intracranial stents, neurovascular thrombectomy devices, embolic protection devices, flow diverters, intrasaccular devices, and others. Embolic coils are small, flexible metal devices used to block blood flow within aneurysms or abnormal vessels, encouraging clot formation to prevent rupture or bleeding. These devices are indicated for conditions such as brain aneurysms and strokes and are utilized through techniques like neurothrombectomy, cerebral angiography, stenting, coiling procedures, and flow disruption. The main end users consist of hospitals, ambulatory surgical centers, specialty clinics, and research institutions.
The revenues for a specified geography are consumption values and are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Neurointerventional Devices Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on neurointerventional devices market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for neurointerventional devices? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The neurointerventional devices market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product: Embolic Coils; Carotid Stents; Intracranial Stents; Neurovascular Thrombectomy; Embolic Protection Device; Flow Diverters Device; Intrasaccular Device; Other Products2) By Indication: Brain Aneurysm; Stroke; Other Indications
3) By Technique: Neurothrombectomy Procedure; Cerebral Angiography; Stenting; Coiling Procedures; Flow Disruption
4) By End User: Hospitals; Ambulatory Surgical Centers; Specialty Clinics; Research Institutes
Subsegments:
1) By Embolic Coils: Bare Platinum Coils; Coated or Modified Coils; Complex-Shaped Coils; Detachable Coils2) By Carotid Stents: Open-Cell Stents; Closed-Cell Stents; Hybrid-Cell Stents; Drug-Eluting Stents
3) By Intracranial Stents: Self-Expanding Stents; Balloon-Expandable Stents; Drug-Eluting Stents
4) By Neurovascular Thrombectomy Devices: Stent Retrievers; Aspiration Catheters; Combined Thrombectomy Systems
5) By Embolic Protection Devices: Distal Filters; Proximal Occlusion Devices; Distal Occlusion Devices
6) By Flow Diverters Devices: Braided Flow Diverters; Dual-Layer Flow Diverters; Coated Flow Diverters
7) By Intrasaccular Devices: Woven Mesh Implants; Expandable Mesh Baskets; Flow Disrupting Implants
8) By Other Products: Microcatheters; Guidewires; Balloon Catheters; Distal Access Catheters
Companies Mentioned: Johnson & Johnson; Abbott Laboratories; Medtronic plc; Stryker Corporation; Boston Scientific Corporation; Terumo Corporation; Kaneka Corporation; Cook Medical LLC; Integra LifeSciences Holdings Corporation; Merit Medical Systems Inc.; MicroPort Scientific Corporation; Penumbra Inc.; Asahi Intecc Co. Ltd.; MicroVention Inc.; Phenox GmbH; Balton Sp. z o.o.; Zylox-Tonbridge Medical Technology Co. Ltd.; Balt Group SAS; Veiva Scientific Co. Ltd.; InspireMD Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain.
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Neurointerventional Devices market report include:- Johnson & Johnson
- Abbott Laboratories
- Medtronic plc
- Stryker Corporation
- Boston Scientific Corporation
- Terumo Corporation
- Kaneka Corporation
- Cook Medical LLC
- Integra LifeSciences Holdings Corporation
- Merit Medical Systems Inc.
- MicroPort Scientific Corporation
- Penumbra Inc.
- Asahi Intecc Co. Ltd.
- MicroVention Inc.
- Phenox GmbH
- Balton Sp. z o.o.
- Zylox-Tonbridge Medical Technology Co. Ltd.
- Balt Group SAS
- Veiva Scientific Co. Ltd.
- InspireMD Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | October 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 2.76 Billion |
| Forecasted Market Value ( USD | $ 3.65 Billion |
| Compound Annual Growth Rate | 7.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |