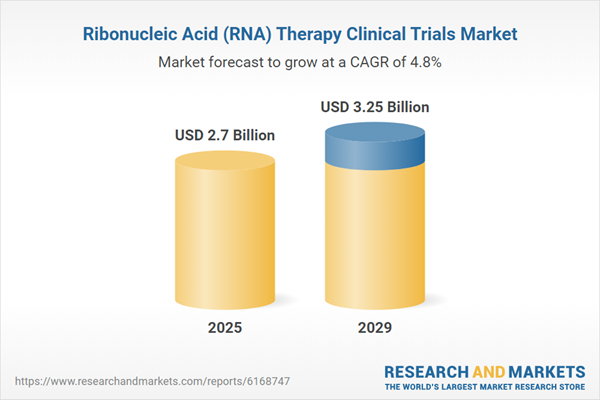
The ribonucleic acid (RNA) therapy clinical trials market size is expected to see steady growth in the next few years. It will grow to $3.25 billion in 2029 at a compound annual growth rate (CAGR) of 4.8%. The growth expected in the forecast period can be attributed to the increasing prevalence of rare diseases, rising regulatory approvals, growing investment in clinical research, increased patient participation, and a heightened focus on personalized medicine. Key trends during this period include advancements in RNA delivery systems, progress in biomarker-driven trial designs, integration of artificial intelligence in trial design, adoption of technology in decentralized clinical trials, and developments in regulatory fast-track pathways.
The growing burden of infectious diseases is expected to drive the expansion of the ribonucleic acid (RNA) therapy clinical trials market in the coming years. Infectious diseases result from pathogenic microorganisms such as bacteria, viruses, fungi, or parasites that invade the body, multiply, and cause health problems. The increase in infectious diseases is largely attributed to the rise in global travel, which accelerates the spread of pathogens across different regions and populations. RNA therapy clinical trials play a crucial role in addressing infectious diseases by facilitating the development and testing of targeted treatments that can quickly adapt to new pathogens. For example, in April 2025, a report by the Minnesota Department of Health, a US-based state agency, indicated that the proportion of HIV cases among males aged 35 to 39 rose from 10% in 2023 to 17% in 2024. Hence, the increasing burden of infectious diseases is propelling the growth of the RNA therapy clinical trials market.
Leading companies in the RNA therapy clinical trials market are concentrating on developing innovative approaches, such as RNA-based antisense oligonucleotide therapy clinical trials, which target specific genetic sequences to regulate gene expression and provide highly precise treatments for genetic and rare diseases. RNA-based antisense oligonucleotide therapy clinical trials investigate the use of short synthetic RNA strands designed to bind specific messenger RNA targets to modulate gene expression and treat genetic disorders. For instance, in December 2024, Sepul Bio, a biotechnology company based in France, started the LUNA Phase 2b clinical trial to assess ultevursen, an RNA-based antisense oligonucleotide therapy aimed at mutations in exon 13 of the USH2A gene responsible for retinitis pigmentosa (RP) and Usher syndrome type 2a. This two-year, double-masked, randomized, sham-controlled trial plans to enroll 81 adults and children aged eight and older at multiple sites worldwide. The therapy targets individuals with RP or non-syndromic RP linked to exon 13 mutations in USH2A, confirmed through genetic testing. The LUNA trial follows encouraging outcomes from earlier Phase 1/2 studies where ultevursen demonstrated improvements in visual acuity, retinal sensitivity, and retinal structure.
In July 2023, Novartis AG, a pharmaceutical company based in Switzerland, acquired DTx Pharma for $1 billion. This acquisition is intended to enhance Novartis’s neuroscience pipeline and broaden its expertise in RNA-based therapeutics by incorporating DTx Pharma’s FALCON platform and advancing innovative small interfering RNA (siRNA) programs targeting neuromuscular and neurological disorders. DTx Pharma is a US-based company specializing in RNA-based therapeutic clinical trials for neuromuscular and neurological diseases.
Major players in the ribonucleic acid (rna) therapy clinical trials market are Pfizer Inc., AstraZeneca plc, Novartis AG, GlaxoSmithKline plc, Moderna Inc., BioNTech SE, Biogen Inc., Daiichi Sankyo Company Limited, Charles River Laboratories International Inc., Alnylam Pharmaceuticals Inc., Sarepta Therapeutics Inc., Ionis Pharmaceuticals Inc., Arrowhead Pharmaceuticals Inc., Arcturus Therapeutics Holdings Inc., Cartesian Therapeutics Inc., CureVac N.V., Orna Therapeutics Inc., Silence Therapeutics plc, ETHRIS GmbH, ProQR Therapeutics N.V., Lexeo Therapeutics Inc., Avidity Biosciences Inc., Aro Biotherapeutics Company, Wave Life Sciences Ltd.
North America was the largest region in the ribonucleic acid (RNA) therapy clinical trials market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in ribonucleic acid (RNA) therapy clinical trials report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the ribonucleic acid (RNA) therapy clinical trials market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The ribonucleic acid (RNA) therapy clinical trials market includes revenues earned by entities by providing services, such as RNA therapeutic administration, biological sample collection and analysis, long-term follow-up, genetic and molecular testing, and patient screening and enrollment. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the consequent trade frictions in spring 2025 are severely impacting the pharmaceutical companies contend with tariffs on APIs, glass vials, and lab equipment inputs with few alternative sources. Generic drug makers, operating on razor-thin margins, are especially vulnerable, with some reducing production of low-profit medicines. Biotech firms face delays in clinical trials due to tariff-related shortages of specialized reagents. In response, the industry is expanding API production in India and Europe, increasing inventory stockpiles, and pushing for trade exemptions for essential medicines.
The ribonucleic acid (RNA) therapy clinical trials market research report is one of a series of new reports that provides ribonucleic acid (RNA) therapy clinical trials market statistics, including ribonucleic acid (RNA) therapy clinical trials industry global market size, regional shares, competitors with a ribonucleic acid (RNA) therapy clinical trials market share, detailed ribonucleic acid (RNA) therapy clinical trials market segments, market trends and opportunities, and any further data you may need to thrive in the ribonucleic acid (RNA) therapy clinical trials industry. This ribonucleic acid (RNA) therapy clinical trials market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Ribonucleic acid (RNA) therapy clinical trials are research studies conducted to assess the safety, efficacy, and optimal application of therapies using RNA-based molecules to treat or prevent diseases. These trials focus on developing innovative medical treatments that work by modulating gene expression, correcting genetic defects, or enabling the body to produce beneficial proteins to address various health conditions.
The main modalities in RNA therapy clinical trials include RNA interference, antisense therapy, messenger RNA (mRNA), oligonucleotides, non-antisense, and non-RNA interference (RNAi) approaches. RNA interference is a natural cellular mechanism where small RNA molecules suppress gene expression or translation by neutralizing specific messenger RNA molecules. These trials cover multiple clinical phases - phase I, phase II, phase III, and phase IV - and target a broad range of therapeutic areas such as rare diseases, anti-infectives, cancer, neurological disorders, metabolic and alimentary conditions, musculoskeletal diseases, cardiovascular and respiratory ailments, sensory disorders, and more.
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Ribonucleic Acid (RNA) Therapy Clinical Trials Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on ribonucleic acid (rna) therapy clinical trials market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for ribonucleic acid (rna) therapy clinical trials? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The ribonucleic acid (rna) therapy clinical trials market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Modality: Ribonucleic Acid (RNA) Interference; Antisense Therapy; Messenger Ribonucleic Acid (RNA); Oligonucleotide; Non-Antisense; Non-Ribonucleic Acid Interference (RNAi)2) By Clinical Trials Phase: Phase I; Phase II; Phase III; Phase IV
3) By Therapeutic Areas: Rare Diseases; Anti-Infective; Anticancer; Neurological; Alimentary or Metabolic; Musculoskeletal; Cardiovascular Respiratory; Sensory; Other Therapeutic Areas
Subsegments:
1) By Ribonucleic Acid (RNA) Interference: Small Interfering RNA (siRNA) Therapy Trials; MicroRNA (miRNA)-Based Therapy Trials; Short Hairpin RNA (shRNA) Therapy Trials; Dicer Substrate RNA Therapy Trials2) By Antisense Therapy: Gapmer Antisense Oligonucleotide Trials; Steric Blocking Antisense Trials; Splice-Switching Antisense Oligonucleotide Trials; Antisense Oligonucleotide-Conjugated Delivery Trials
3) By Messenger Ribonucleic Acid (RNA): mRNA-Based Vaccines Trials; Self-Amplifying mRNA (saRNA) Therapy Trials; Non-Replicating mRNA Therapy Trials; Circular mRNA Therapy Trials
4) By Oligonucleotide: Phosphorothioate Oligonucleotide Trials; Peptide Nucleic Acid (PNA) Trials; Locked Nucleic Acid (LNA) Trials; Morpholino Oligonucleotide Trials
5) By Non-Antisense: RNA Aptamer Therapy Trials; Guide RNA (gRNA)-Mediated CRISPR Trials; RNA Editing Therapy Trials; RNA Scaffolding or Regulatory RNA Therapy Trials
6) By Non-Ribonucleic Acid Interference (RNAi): Ribozymes Therapy Trials; RNA Decoys or Sponge RNA Trials; Long Non-Coding RNA (lncRNA) Therapy Trials; Small Activating RNA (saRNA) Therapy Trials
Companies Mentioned: Pfizer Inc.; AstraZeneca plc; Novartis AG; GlaxoSmithKline plc; Moderna Inc.; BioNTech SE; Biogen Inc.; Daiichi Sankyo Company Limited; Charles River Laboratories International Inc.; Alnylam Pharmaceuticals Inc.; Sarepta Therapeutics Inc.; Ionis Pharmaceuticals Inc.; Arrowhead Pharmaceuticals Inc.; Arcturus Therapeutics Holdings Inc.; Cartesian Therapeutics Inc.; CureVac N.V.; Orna Therapeutics Inc.; Silence Therapeutics plc; ETHRIS GmbH; ProQR Therapeutics N.V.; Lexeo Therapeutics Inc.; Avidity Biosciences Inc.; Aro Biotherapeutics Company; Wave Life Sciences Ltd.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain.
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Ribonucleic Acid (RNA) Therapy Clinical Trials market report include:- Pfizer Inc.
- AstraZeneca plc
- Novartis AG
- GlaxoSmithKline plc
- Moderna Inc.
- BioNTech SE
- Biogen Inc.
- Daiichi Sankyo Company Limited
- Charles River Laboratories International Inc.
- Alnylam Pharmaceuticals Inc.
- Sarepta Therapeutics Inc.
- Ionis Pharmaceuticals Inc.
- Arrowhead Pharmaceuticals Inc.
- Arcturus Therapeutics Holdings Inc.
- Cartesian Therapeutics Inc.
- CureVac N.V.
- Orna Therapeutics Inc.
- Silence Therapeutics plc
- ETHRIS GmbH
- ProQR Therapeutics N.V.
- Lexeo Therapeutics Inc.
- Avidity Biosciences Inc.
- Aro Biotherapeutics Company
- Wave Life Sciences Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | October 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 2.7 Billion |
| Forecasted Market Value ( USD | $ 3.25 Billion |
| Compound Annual Growth Rate | 4.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |