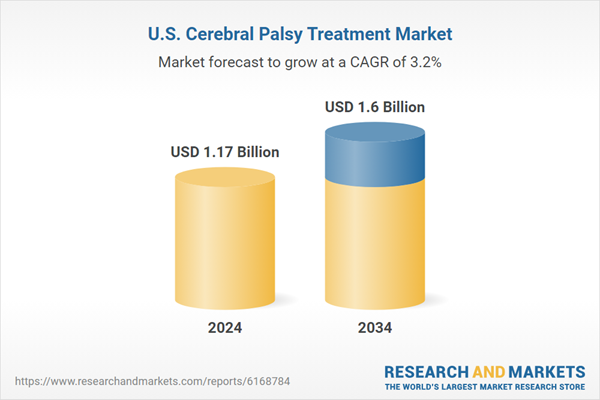
This projected growth is fueled by a rising number of diagnosed cases nationwide, ongoing advancements in drug development, and expanding research investments. Increasing public and clinical focus on early diagnosis and supportive policies around reimbursement are also playing a central role. As more patients gain access to innovative therapies, the market continues to benefit from a growing commitment among healthcare providers and institutions to improve quality of life and motor function outcomes in individuals living with cerebral palsy through more individualized, multidisciplinary treatment options.
Treatment for cerebral palsy in the U.S. spans a combination of therapeutic, surgical, pharmaceutical, and support-based approaches aimed at enhancing movement, managing symptoms, and improving daily living. Advances in oral drug therapies, injectable treatments, and assistive technologies are providing more tailored care strategies. Patients often undergo a combination of physical, speech, and occupational therapies, use assistive mobility tools, and receive medications such as muscle relaxants or undergo surgical interventions. These individualized treatment programs are developed to support greater independence and long-term symptom management, though the underlying condition itself is not curable.
The muscle relaxants segment held a 58.7% share in 2024, driven by their effectiveness in treating spasticity. Drugs such as diazepam, baclofen, and tizanidine are frequently used to reduce muscle stiffness and are often administered prior to orthopedic or neurosurgical procedures. These medications are critical components in rehabilitation regimens, supporting post-surgical recovery and long-term symptom relief. Patients typically access these drugs through a combination of hospital-based care, retail pharmacy options, and specialized channels for advanced delivery systems like intrathecal pumps.
The mixed type cerebral palsy segment is projected to grow at a CAGR of 3.8% through 2034. Individuals diagnosed with this form of CP experience overlapping symptoms, including spasticity, uncontrolled movements, and coordination challenges. Because of this complexity, treatment plans for mixed cerebral palsy increasingly rely on personalized pharmaceutical strategies. These include a blend of muscle relaxants, oral medications, and targeted injectables, often accompanied by surgical or rehabilitative therapies. This approach aligns with the increasing clinical shift toward customized treatment plans that cater to each patient’s unique presentation of symptoms.
South Atlantic Cerebral Palsy Treatment Market held a 24.5% share in 2024. This dominance is linked to the presence of a robust support ecosystem made up of community groups, advocacy organizations, and regional care networks that promote early intervention and long-term management strategies. These networks actively promote public education, support fundraising for research, and help families better navigate the complex landscape of cerebral palsy treatments. Their efforts have helped improve patient access to care and encourage informed decision-making across the treatment journey.
Key companies active in the U.S. Cerebral Palsy Treatment Market include Teva, Novartis, AbbVie, Merz Pharmaceuticals, Amneal, UCB, GSK, Roche, CHEPLAPHARM, Dr. Reddy’s, IPSEN, and VIATRIS. To strengthen their foothold in the U.S. cerebral palsy treatment landscape, leading companies are investing in clinical research to develop next-generation formulations with improved tolerability and long-acting efficacy. Many are also exploring innovative delivery systems such as infusion pumps and sustained-release drugs that improve compliance and reduce dosing frequency. Strategic alliances with hospitals and rehabilitation centers enable broader product integration into care protocols. Additionally, companies are expanding their reimbursement support programs and digital patient engagement platforms to enhance access and education for families and caregivers managing long-term treatment plans.
Comprehensive Market Analysis and Forecast
- Industry trends, key growth drivers, challenges, future opportunities, and regulatory landscape
- Competitive landscape with Porter’s Five Forces and PESTEL analysis
- Market size, segmentation, and regional forecasts
- In-depth company profiles, business strategies, financial insights, and SWOT analysis
This product will be delivered within 2-4 business days.
Table of Contents
Companies Mentioned
The key companies profiled in this U.S. Cerebral Palsy Treatment market report include:- AbbVie
- amneal
- CHEPLAPHARM
- Dr. Reddy's
- GSK
- IPSEN
- Merz Pharmaceuticals
- Novartis
- Roche
- Teva
- UCB
- VIATRIS
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | August 2025 |
| Forecast Period | 2024 - 2034 |
| Estimated Market Value ( USD | $ 1.17 Billion |
| Forecasted Market Value ( USD | $ 1.6 Billion |
| Compound Annual Growth Rate | 3.2% |
| Regions Covered | United States |
| No. of Companies Mentioned | 13 |