Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
The growth of this market in the U.S. is driven by several factors, including the rising prevalence of cardiovascular diseases, an aging population, and increasing awareness of advanced treatment options that offer improved patient outcomes. However, the market faces challenges including high device costs and the need for skilled operators to perform complex procedures. Despite these barriers, ongoing research and development, along with growing clinical evidence supporting the benefits of catheter-based electrophysiology interventions, are expected to drive robust growth.
Key Market Drivers
Growth in Healthcare Industry
In 2023, U.S. healthcare spending grew by 7.5%, reaching USD 4.9 trillion, or approximately USD 14,570 per person. This expenditure accounted for 17.6% of the country’s Gross Domestic Product (GDP).The robust growth of the healthcare industry in the United States is a key driver fueling the expansion of the electrophysiology (EP) mapping and ablation devices market. Increasing prevalence of cardiovascular diseases, particularly arrhythmias, combined with rising patient awareness and demand for minimally invasive treatment options, has amplified the need for advanced EP technologies.Moreover, rising investments by hospitals and clinics in state-of-the-art electrophysiology labs enable the adoption of sophisticated mapping and ablation devices. Since 2006, private equity has invested nearly USD 1 trillion in U.S. healthcare. While representing a small share of the sector, these investments have been crucial funding research on diseases like Alzheimer’s and Parkinson’s, expanding and upgrading facilities, modernizing medical records and data, and supporting other essential improvements. As healthcare providers focus on improving patient outcomes and reducing procedural risks, the demand for innovative EP solutions continues to accelerate, making the expanding healthcare industry a critical growth enabler for this market.
Key Market Challenges
Shortage of Skilled Electrophysiologists
A significant challenge facing the United States Electrophysiology Mapping and Ablation Devices Market is the shortage of skilled electrophysiologists. The complexity of electrophysiology procedures demands highly trained and experienced specialists to accurately interpret mapping data and perform precise ablation treatments. However, the current supply of qualified electrophysiologists is insufficient to meet the growing demand driven by the rising prevalence of cardiac arrhythmias and expanding adoption of advanced mapping and ablation technologies.This shortage creates operational bottlenecks in healthcare facilities, potentially leading to longer patient wait times, delayed treatments, and underutilization of sophisticated electrophysiology equipment. Furthermore, the limited availability of skilled professionals can increase procedural risks and impact overall treatment outcomes, which in turn affects market growth and device adoption.
Key Market Trends
Growing Use of Wearable Monitoring Devices
The increasing adoption of wearable monitoring devices is emerging as a significant trend within the U.S. electrophysiology mapping and ablation devices market. According to the 2023 Rock Health Digital Health Consumer Adoption Survey, 44% of Americans use wearable health tracking devices, including smartwatches and smart rings, to monitor various health metrics ranging from sleep patterns to heart rate. These devices enable continuous, real-time cardiac monitoring, allowing for early detection and precise diagnosis of arrhythmias outside traditional clinical settings.Enhanced patient convenience and improved data accuracy offered by wearables facilitate better treatment planning and timely intervention, which complements electrophysiology mapping and ablation procedures. Integration of wearable technology with advanced analytics and remote monitoring platforms is driving innovation and expanding the scope of cardiac care. As both healthcare providers and patients prioritize proactive disease management, the growing use of wearable monitoring devices is expected to substantially influence market dynamics, promoting more personalized and efficient electrophysiological treatments.
Key Market Players
- Siemens Healthineers
- Koninklijke Philips N.V.
- Abbott Laboratories
- Johnson & Johnson Services, Inc.
- GE HealthCare
- Boston Scientific Corporation
- Medtronic Plc
- Biotronik SE & Co KG
- MicroPort Scientific Corporation
- CardioFocus, Inc.
Report Scope
In this report, the United States Electrophysiology Mapping And Ablation Devices Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:United States Electrophysiology Mapping And Ablation Devices Market, By Procedure Type:
- EP Ablation Procedures
- Stand-Alone EP Diagnostic Procedures
- Others
United States Electrophysiology Mapping And Ablation Devices Market, By Product:
- Mapping & Lab Systems
- Diagnostic Catheters
- Ablation Catheters
- Accessory Devices
- Others
United States Electrophysiology Mapping And Ablation Devices Market, By Region:
- Northeast
- Midwest
- South
- West
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the United States Electrophysiology Mapping And Ablation Devices Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The leading companies profiled in this United States Electrophysiology Mapping And Ablation Devices market report include:- Siemens Healthineers
- Koninklijke Philips N.V.
- Abbott Laboratories
- Johnson & Johnson Services, Inc.
- GE HealthCare
- Boston Scientific Corporation
- Medtronic Plc
- Biotronik SE & Co KG
- MicroPort Scientific Corporation
- CardioFocus, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 85 |
| Published | September 2025 |
| Forecast Period | 2024 - 2030 |
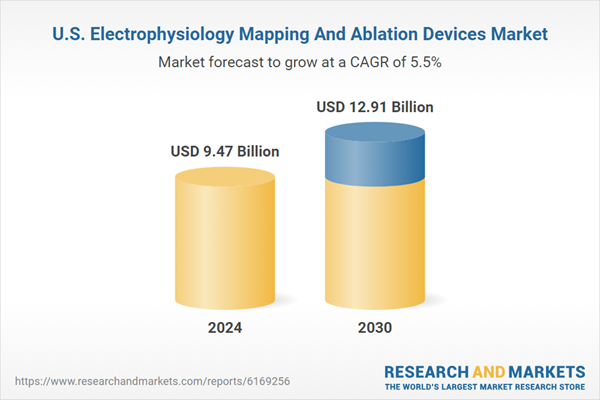
| Estimated Market Value ( USD | $ 9.47 Billion |
| Forecasted Market Value ( USD | $ 12.91 Billion |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | United States |
| No. of Companies Mentioned | 10 |