1. PREFACE
1.1. Scope of the Report
1.2. Research Methodology
1.2.1. Research Assumptions
1.2.2. Project Methodology
1.2.3. Forecast Methodology
1.2.4. Robust Quality Control
1.2.5. Key Considerations
1.2.5.1. Demographics
1.2.5.2. Economic Factors
1.2.5.3. Government Regulations
1.2.5.4. Supply Chain
1.2.5.5. COVID Impact / Related Factors
1.2.5.6. Market Access
1.2.5.7. Healthcare Policies
1.2.5.8. Industry Consolidation
1.3 Key Questions Answered
1.4. Chapter Outlines
3. INTRODUCTION
3.1. Chapter Overview
3.2. Concept of Microbiota and Microbiome
3.2.1. Discovery of the Human Microbiome
3.2.2. Functions of the Human Microbiome
3.3. Overview of Gut Flora
3.3.1. Role of Gut Flora in Human Health
3.3.2. Factors Affecting Gut Flora
3.3.2.1. Antibiotic Consumption
3.3.2.2. Age and Pregnancy
3.3.2.2.1. Mode of Childbirth
3.3.2.2.2. Type of Feeding
3.3.2.2.3. Antibiotic Consumption by Mother
3.3.2.3. Stress-related Factors
3.3.2.4. Dietary Factors
3.3.2.5. Impact of Lifestyle
3.4. Microbiome and Associated Diseases
3.4.1. Cancer
3.4.2. Inflammatory Bowel Disease (IBD)
3.4.3. Obesity
3.4.4. Parkinson’s Disease
3.4.5. Type 2 Diabetes
3.4.6. Other Disease Indications
3.5. Impact of Microbiota on Drug Pharmacokinetics
3.6. Impact of Microbiota on Therapeutic Outcomes
3.7. Microbiome Therapeutics
3.7.1. Probiotics
3.7.1.1. Beneficial Bacterial Strains
3.7.1.1.1. Lactobacilli
3.7.1.1.2. Bifidobacteria
3.7.1.1.3. Others
3.7.1.2. Key Therapeutic Areas
3.7.1.2.1. Antibiotic-Associated Diarrhea (AAD)
3.7.1.2.2. Bacterial Vaginosis
3.7.1.2.3. High Blood Pressure
3.7.1.2.4. Hypercholesterolemia
3.7.1.2.5. Infectious Childhood Diarrhea (ICD)
3.7.1.2.6. Inflammatory Bowel Disease (IBD)
3.7.1.2.7. Lactose Intolerance
3.7.1.2.8. Vitamin Production
3.7.1.2.9. Weight Management
3.7.1.3. Side Effects of Probiotics
3.7.2. Prebiotics
3.7.2.1. Sources of Prebiotics
3.7.2.2. Types of Prebiotics
3.7.2.2.1. Fructo-Oligosaccharides (FOS)
3.7.2.2.2. Galacto-Oligosaccharides (GOS)
3.7.2.2.3. Inulin
3.7.2.3. Key Therapeutic Areas
3.7.2.3.1. Antibiotic Associated Diarrhea (AAD)
3.7.2.3.2. Constipation
3.7.2.3.3. Gastrointestinal Diseases
3.7.2.3.4. Dysbiosis
3.7.2.4. Side Effects of Prebiotics
3.8. The Human Microbiome Project (HMP)
3.8.1. Project Approach
3.8.2. Project Initiatives
3.8.3. Project Achievements
3.9. Regulatory Guidelines for Live Biotherapeutic Products (LBPs)
3.10. Key Challenges Associated with the Development of Microbiome Therapeutics
3.11. Future Perspectives
4. MICROBIOME THERAPEUTICS: MARKET LANDSCAPE
4.1. Chapter Overview
4.2. Microbiome Therapeutics: Clinical Pipeline
4.2.1. Analysis by Phase of Development
4.2.2. Analysis by Type of Molecule
4.2.3. Analysis by Phase of Development and Type of Molecule
4.2.4. Analysis by Type of Biologic
4.2.5. Analysis by Type of Product
4.2.6. Analysis by Target Indication
4.2.7. Analysis by Therapeutic Area
4.2.8. Analysis by Route of Administration
4.2.9. Analysis by Type of Formulation
4.2.10. Analysis by Dose Frequency
4.2.11. Analysis by Type of Therapy
4.2.12. Analysis by Combination Drug
4.3. Microbiome Therapeutics: Preclinical Pipeline
4.3.1. Analysis by Phase of Development
4.3.2. Analysis by Type of Molecule
4.3.3. Analysis by Phase of Development and Type of Molecule
4.3.4. Analysis by Type of Biologic
4.3.5. Analysis by Type of Product
4.3.6. Analysis by Target Indication
4.3.7. Analysis by Therapeutic Area
4.3.8. Analysis by Route of Administration
4.4. Microbiome Therapeutics: List of Drug Developers
4.4.1. Analysis by Year of Establishment
4.4.2. Analysis by Company Size
4.4.3. Analysis by Location of Headquarters
4.4.4. Analysis by Company Size and Location of Headquarters
5. MICROBIOME THERAPEUTICS: COMPANY AND DRUG PROFILES
5.1. Chapter Overview
5.2. Microbiome Therapeutics Developers: Companies with Candidate(s) in Highest Phase of Development
5.3. Finch Therapeutics
5.3.1. Company Overview
5.3.2. Microbiome-Based Product Portfolio
5.3.2.1. CP101
5.3.2.1.1. Drug Overview
5.3.2.1.2. Current Status of Development
5.3.2.1.3. Clinical Studies
5.3.3. Recent Developments and Future Outlook
5.4. Infant Bacterial Therapeutics
5.4.1. Company Overview
5.4.2. Microbiome-Based Product Portfolio
5.4.2.1. IBP-9414
5.4.2.1.1. Drug Overview
5.4.2.1.2. Current Status of Development
5.4.2.1.3. Clinical Studies
5.4.3. Recent Developments and Future Outlook
5.5. MaaT Pharma
5.5.1. Company Overview
5.5.2. Microbiome-Based Product Portfolio
5.5.2.1. MaaT013
5.5.2.1.1. Drug Overview
5.5.2.1.2. Current Status of Development
5.5.2.1.3. Clinical Studies
5.5.3. Recent Developments and Future Outlook
5.6. OxThera
5.6.1. Company Overview
5.6.2. Microbiome-Based Product Portfolio
5.6.2.1. Oxabact
5.6.2.1.1. Drug Overview
5.6.2.1.2. Current Status of Development
5.6.2.1.3. Clinical Studies
5.6.3. Recent Developments and Future Outlook
5.7. Rebiotix (acquired by Ferring Pharmaceuticals)
5.7.1. Company Overview
5.7.2. Financial Information
5.7.3. Microbiome-Based Product Portfolio
5.7.3.1. RBX2660
5.7.3.1.1. Drug Overview
5.7.3.1.2. Current Status of Development
5.7.3.1.3. Clinical Studies
5.7.4. Recent Developments and Future Outlook
5.8. Seres Therapeutics
5.8.1. Company Overview
5.8.2. Financial Information
5.8.3. Microbiome-Based Product Portfolio
5.8.3.1. SER-109
5.8.3.1.1. Drug Overview
5.8.3.1.2. Current Status of Development
5.8.3.1.3. Clinical Studies
5.8.3.1.4. Clinical Trial End-Point Analysis
5.9. Microbiome Therapeutics Developers: Companies with Maximum Number of Therapeutic Programs
5.10. 4D Pharma
5.10.1. Company Overview
5.10.2. Financial Information
5.10.3. Microbiome-based Drug Portfolio
5.10.4. Recent Developments and Future Outlook
5.11. Biosortia Pharmaceuticals
5.11.1. Company Overview
5.11.2. Microbiome-based Drug Portfolio
5.11.3. Recent Developments and Future Outlook
5.12. Qu Biologics
5.12.1. Company Overview
5.12.2. Microbiome-based Drug Portfolio
5.12.3. Recent Developments and Future Outlook
5.13. Servatus
5.13.1. Company Overview
5.13.2. Microbiome-based Drug Portfolio
5.13.3. Recent Developments and Future Outlook
6. CLINICAL TRIAL ANALYSIS: HUMAN MICROBIOME THERAPEUTICS
6.1. Chapter Overview
6.2. Human Microbiome Therapeutics: List of Clinical Trials
6.2.1. Analysis by Trial Status
6.2.2. Analysis by Trial Registration Year
6.2.3. Analysis by Trial Registration Year and Type of Study
6.2.4. Analysis by Trial Phase and Trial Status
6.2.5. Year-wise Trend of Completed and Recruiting Trials
6.2.6. Analysis by Study Design
6.2.7. Analysis by Patient Enrollment
6.2.8. Analysis by Age Category
6.2.9. Analysis by Type of Sponsor / Collaborator
6.2.10. Most Active Industry Players: Analysis by Number of Registered Trials
6.2.11. Most Active Non-Industry Players: Analysis by Number of Registered Trials
6.2.12. Analysis by Trial Location
6.2.13. Analysis by Trial Status and Geography
7. MICROBIOME DIAGNOSTICS AND SCREENING / PROFILING TESTS: MARKET LANDSCAPE
7.1. Chapter Overview
7.2. Overview of Microbiome Diagnostics and Screening / Profiling Tests
7.3. Microbiome Diagnostics and Screening / Profiling Tests: Marketed and Under Development Products
7.3.1. Analysis by Stage of Development
7.3.2. Analysis by Type of Test
7.3.3. Analysis by Stage of Development and Type of Test
7.3.4. Analysis by Type of Sample Analyzed
7.3.5. Analysis by Type of Screening Technique
7.3.6. Analysis by Target Indication
7.3.7. Analysis by Therapeutic Area
7.3.8. Analysis by Purpose of Test
7.4. Microbiome Diagnostic and Screening / Profiling Tests Providers
7.4.1. Analysis by Year of Establishment
7.4.2. Analysis by Company Size
7.4.3. Analysis by Location of Headquarters
7.4.4. Analysis by Company Size and Location of Headquarters
8. MICROBIOME DIAGNOSTIC AND SCREENING / PROFILING TEST PROVIDERS: COMPANY PROFILES
8.1. Chapter Overview
8.2. Shoreline Biome
8.2.1. Company Overview
8.2.2. Microbiome Test Portfolio
8.2.3. Recent Developments and Future Outlook
8.3. DNA Genotek
8.3.1. Company Overview
8.3.2. Microbiome Test Portfolio
8.3.3. Recent Developments and Future Outlook
8.4. Invivo Healthcare
8.4.1. Company Overview
8.4.2. Microbiome Test Portfolio
8.4.3. Recent Developments and Future Outlook
8.5. GoodGut
8.5.1. Company Overview
8.5.2. Microbiome Test Portfolio
8.5.3. Recent Developments and Future Outlook
8.6. BiomeDx
8.6.1. Company Overview
8.6.2. Microbiome Test Portfolio
8.6.3. Recent Developments and Future Outlook
9. FECAL MICROBIOTA THERAPY (FMT)
9.1. Chapter Overview
9.2. Introduction to Fecal Microbiota Therapies (FMT)
9.3. Historical Overview
9.4. Fecal Microbiota Therapies: Procedure and Clinical Relevance
9.4.1. Donor Selection
9.4.2. Administration Procedure
9.4.3. Routes of Administration
9.4.4. Consequences and Adverse Events
9.4.5. Clinical Guidelines Associated with FMT
9.5. Regulatory Guidelines Related to Fecal Microbiota Therapies
9.6. Insurance Coverage for Fecal Microbiota Therapies
9.7. Fecal Microbiota Therapies: Marketed and Development Pipeline
9.7.1. Analysis by Application Area
9.7.2. Analysis by Status of Development
9.7.3. Analysis by Target Indication
9.7.4. Analysis by Therapeutic Area
9.7.5. Analysis by Route of Administration
9.7.6. Fecal Microbiota Therapies: List of Developers
9.7.6.1. Analysis by Year of Establishment
9.7.6.2. Analysis by Company Size
9.7.6.3. Analysis by Location of Headquarters
10. CLINICAL TRIALS ANALYSIS: FECAL MICROBIOTA THERAPY
10.1. Chapter Overview
10.2. Scope and Methodology
10.3. Fecal Microbiota Therapies: Clinical Trial Analysis
10.3.1. Analysis by Trial Status
10.3.2. Analysis by Trial Registration Year
10.3.3. Analysis by Trial Recruitment Status
10.3.4. Analysis by Trial Phase and Number of Patients Enrolled
10.3.5. Analysis by Study Design
10.3.6. Leading Industry Players: Analysis by Number of Registered Trials
10.3.7. Leading Non-industry Players: Analysis by Number of Registered Trials
10.3.8. Analysis by Trial Location
10.3.9. Analysis by Trial Status and Geography
11. ATTRACTIVENESS COMPETITIVENESS (AC) MATRIX
11.1. Chapter Overview
11.2. AC Matrix: An Overview
11.2.1. Strong Business Units
11.2.2. Average Business Units
11.2.3. Weak Business Units
11.3. AC Matrix: Analytical Methodology
11.4. AC Matrix: Plotting the Information
11.5. AC Matrix: Analyzing the Data
11.5.1. Strong Business Units
11.5.2. Average Business Units
11.5.3. Weak Business Units
11.6. Concluding Remarks
12. MICROBIOME RELATED INITIATIVES OF BIG PHARMA PLAYERS
12.1. Chapter Overview
12.2. Scope and Methodology
12.3. Initiatives of Big Pharma Players
12.3.1. Analysis by Portfolio Diversity
12.3.2. Analysis by Phase of Development
12.3.3. Analysis by Type of Molecule
12.3.4. Analysis by Type of Therapy
12.3.5. Analysis by Diversity of Therapeutic Areas
12.4. Benchmarking Analysis of Big Pharma Players
13. START-UP HEALTH INDEXING
13.1. Chapter Overview
13.2. Scope and Methodology
13.3. Benchmarking of Start-ups
13.3.1. Analysis by Portfolio Diversity
13.3.2. Analysis by Phase of Development
13.3.3. Analysis by Diversity in Indication
13.3.4. Analysis by Funding Amount
13.3.5. Analysis by Partnership Activity
13.3.6. Start-up Health Indexing: Roots Analysis Perspective
13.3.7. Start-up Health Indexing: Top Five Start-ups
14. KEY THERAPEUTIC AREAS
14.1. Chapter Overview
14.2. Metabolic Disorders
14.2.1. Diabetes
14.2.1.1. Disease Description
14.2.1.2. Associated Health Risks / Complications
14.2.1.3. Epidemiology
14.2.1.4. Disease Diagnosis
14.2.1.5. Current Treatment Options
14.2.1.5.1. Insulin Therapies
14.2.1.5.2. Non-Insulin Therapies
14.2.1.6. Side Effects of Current Treatment Options
14.2.1.7. Microbiome Therapeutics for Diabetes
14.2.2. Lactose Intolerance
14.2.2.1. Disease Description
14.2.2.2. Epidemiology
14.2.2.3. Current Treatment Options
14.2.2.4. Microbiome Therapeutics for Lactose Intolerance
14.2.3. Nonalcoholic Steatohepatitis (NASH)
14.2.3.1. Disease Description
14.2.3.2. Epidemiology
14.2.3.3. Current Treatment Options
14.2.3.4. Microbiome Therapeutics for NASH
14.2.4. Primary Hyperoxaluria
14.2.4.1. Disease Description
14.2.4.2. Epidemiology
14.2.4.3. Current Treatment Options
14.2.4.4. Microbiome Therapeutics for Primary Hyperoxaluria
14.2.5. Obesity
14.2.5.1. Disease Description
14.2.5.2. Epidemiology
14.2.5.3. Current Treatment Options
14.2.5.4. Side Effects of Current Treatment Options
14.2.5.5. Microbiome Therapeutics for Obesity
14.3. Digestive and Gastrointestinal Disorders
14.3.1. Crohn’s Disease
14.3.1.1. Disease Description
14.3.1.2. Epidemiology
14.3.1.3. Current Treatment Options
14.3.1.4. Side Effects of Current Treatment Options
14.3.1.5. Microbiome Therapeutics for Crohn’s Disease
14.3.2. Irritable Bowel Syndrome (IBS)
14.3.2.1. Disease Description
14.3.2.2. Epidemiology
14.3.2.3. Current Treatment Options
14.3.2.4. Microbiome Therapeutics for IBS
14.3.3. Ulcerative Colitis
14.3.3.1. Disease Description
14.3.3.2. Epidemiology
14.3.3.3. Current Treatment Options
14.3.3.4. Side Effects of Current Treatment Options
14.3.3.5. Microbiome Therapeutics for Ulcerative Colitis
14.4. Oncological Indications
14.4.1. Colorectal Cancer
14.4.1.1. Disease Description
14.4.1.2. Epidemiology
14.4.1.3. Current Treatment Options
14.4.1.4. Side Effects of Current Treatments
14.4.1.5. Microbiome Therapeutics for Colorectal Cancer
14.4.2. Lung Cancer
14.4.2.1. Disease Description
14.4.2.2. Epidemiology
14.4.2.3. Current Treatment Options
14.4.2.4. Side Effects of Current Treatment Options
14.4.2.5. Microbiome Therapeutics for Lung Cancer
14.5. Dermatological Disorders
14.5.1. Acne Vulgaris
14.5.1.1. Disease Description
14.5.1.2. Epidemiology
14.5.1.3. Current Treatment Options
14.5.1.4. Side Effects of Current Treatment Options
14.5.1.5. Microbiome Therapeutics for Acne Vulgaris
14.6. Infectious Diseases
14.6.1. Clostridium Difficile Infections (CDIs)
14.6.1.1. Disease Description
14.6.1.2. Epidemiology
14.6.1.3. Disease Diagnosis
14.6.1.4. Current Treatment Options
14.6.1.5. Side Effects of Current Treatment Options
14.6.1.6. Microbiome Therapeutics for CDI
14.6.2. Bacterial Vaginosis
14.6.2.1. Disease Description
14.6.2.2. Epidemiology
14.6.2.3. Current Treatment Options
14.6.2.4. Side Effects of Current Treatment Options
14.6.2.5. Microbiome Therapeutics for Bacterial Vaginosis
15. PARTNERSHIPS AND COLLABORATIONS
15.1. Chapter Overview
15.2. Partnership Models
15.3. Human Microbiome: List of Partnerships and Collaborations
15.4. Analysis by Year of Partnership
15.5. Analysis by Type of Partnership
15.6. Analysis by Year and Type of Partnership
15.7. Analysis by Type of Product
15.8. Analysis by Target Indication
15.9. Analysis by Therapeutic Area
15.10. Analysis by Type of Company
15.11. Analysis by Type of Partner
15.12. Most Active Players: Analysis by Number of Partnerships
15.13. Intercontinental and Intracontinental Agreements
16. FUNDING AND INVESTMENT ANALYSIS
16.1. Chapter Overview
16.2. Types of Funding
16.3. Microbiome Therapeutics and Diagnostics: List of Funding and Investments
16.3.1. Analysis by Year of Investment
16.3.2. Analysis by Amount Invested
16.3.3. Analysis by Type of Funding
16.3.4. Analysis by Type of Company
16.3.5. Analysis by Purpose of Funding
16.3.6. Analysis by Type of Product
16.3.7. Analysis by Target Indication
16.3.8. Analysis by Therapeutic Area
16.3.9. Analysis by Geography
16.3.10. Most Active Players: Analysis by Number of Instances
16.3.11. Most Active Players: Analysis by Amount Invested
16.3.12. Most Active Investors: Analysis by Number of Instances
16.3.13. Funding and Investment Summary
17. CASE STUDY: CONTRACT SERVICES FOR MICROBIOME THERAPEUTICS AND LIVE BIOTHERAPEUTICS
17.1. Chapter Overview
17.2. Manufacturing Microbiome Therapeutics
17.2.1. Key Steps Involved
17.2.2. Associated Challenges
17.2.3. Growing Demand for Contract Manufacturing Services
17.2.4. Contract Manufacturing Organizations (CMOs)
17.2.4.1. Introduction to Contract Manufacturing
17.2.5. Microbiome Therapeutics: List of Contract Manufacturing Providers
17.2.5.1. Analysis by Year of Establishment
17.2.5.2. Analysis by Company Size
17.2.5.3. Analysis by Location of Headquarters
17.2.5.4. Analysis by Scale of Operation
17.2.5.5. Analysis by Type of Product Manufactured
17.2.5.6. Analysis by Type of Formulation
17.2.5.7. Analysis by Scale of Operation and Type of Formulation
17.3. Key Considerations for Selecting a CMO / CRO Partner
18. BIG DATA AND MICROBIOME THERAPEUTICS
18.1. Chapter Overview
18.2. Introduction to Big Data
18.3. Internet of Things (IoT)
18.4. Growing Interest in Big Data: Google Trends Analysis
18.5. Key Application Areas
18.6. Big Data in Microbiome Research
18.6.1. Microbiome Data and Personalized Medicine
18.6.2. Microbiome-related Data Management Challenges
18.6.3. National Microbiome Data Center
18.7. Big Data Services for Microbiome Research: List of Companies
18.8. Big Data Services for Microbiome Research: Profiles of Key Players
18.8.1. Human Longevity
18.8.1.1. Company Overview
18.8.1.2. Technology and Service Portfolio
18.8.1.3. Recent Developments and Future Outlook
18.8.2. Resilient Biotics
18.8.2.1. Company Overview
18.8.2.2. Technology and Service Portfolio
18.8.2.3. Recent Developments and Future Outlook
18.8.3. Resphera Biosciences
18.8.3.1. Company Overview
18.8.3.2. Technology and Service Portfolio
18.8.3.3. Recent Developments and Future Outlook
19. MICROBIOME THERAPEUTICS: MARKET FORECAST AND OPPORTUNITY ANALYSIS
19.1. Chapter Overview
19.2. Key Assumptions
19.3. Forecast Methodology
19.4. Global Microbiome Therapeutics Market, Till 2035
19.4.1. Microbiome Therapeutics Market: Distribution by Type of Product Till 2035
19.4.1.1. Microbiome Therapeutics Market for Probiotic Drugs, Till 2035
19.4.1.2. Microbiome Therapeutics Market for Other Drugs, Till 2035
19.4.2. Microbiome Therapeutics Market: Distribution by Target Disease Indication, Till 2035
19.4.2.1. Microbiome Therapeutics Market for Graft Versus Host Disease, Till 2035
19.4.2.2. Microbiome Therapeutics Market for Necrotizing Enterocolitis, Till 2035
19.4.2.3. Microbiome Therapeutics Market for Primary Hyperoxaluria, Till 2035
19.4.2.4. Microbiome Therapeutics Market for Recurrent C. difficile Infection, Till 2035
19.4.3. Microbiome Therapeutics Market: Distribution by Therapeutic Area, Till 2035
19.4.3.1. Microbiome Therapeutics Market for Digestive and Gastrointestinal Disorders, Till 2035
19.4.3.2. Microbiome Therapeutics Market for Infectious Diseases, Till 2035
19.4.3.3. Microbiome Therapeutics Market for Rare Disorders, Till 2035
19.4.4. Microbiome Therapeutics Market: Route of Administration, Till 2035
19.4.4.1. Microbiome Therapeutics Market for Oral Therapeutics, Till 2035
19.4.4.2. Microbiome Therapeutics Market for Rectal Therapeutics, Till 2035
19.4.5. Microbiome Therapeutics Market: Distribution by Type of Formulation, Till 2035
19.4.5.1. Microbiome Therapeutics Market for Capsules, Till 2035
19.4.5.2. Microbiome Therapeutics Market for Suspensions, Till 2035
19.4.5.3. Microbiome Therapeutics Market for Enemas, Till 2035
19.4.6. Microbiome Therapeutics Market: Distribution by Key Geographical Regions, Till 2035
19.4.6.1. Microbiome Therapeutics Market in North America, Till 2035
19.4.6.2. Microbiome Therapeutics Market in Europe, Till 2035
19.4.6.3. Microbiome Therapeutics Market in Asia-Pacific and Rest of the World, Till 2035
19.4.7. Microbiome Therapeutics Market: Distribution by Leading Drug Developers, Till 2035
19.4.8. Microbiome Therapeutics Market: Distribution by Leading Therapeutic Products, Till 2035
19.4.8.1. SER-109 (Seres Therapeutics)
19.4.8.2. RBX2660 (ReBiotix)
19.4.8.3. CP101 (Finch Therapeutics)
19.4.8.4. IBP-9414 (Infant bacterial Therapeutics)
19.4.8.5. Oxabact (OxThera)
19.4.8.6. MaaT013 (MaaT Pharma)
20. MICROBIOME DIAGNOSTICS: MARKET FORECAST AND OPPORTUNITY ANALYSIS
20.1. Chapter Overview
20.2. Scope and Limitations
20.3. Forecast Methodology
20.4. Global Human Microbiome Diagnostic Test Market, Till 2035
20.4.1. Human Microbiome Diagnostic Tests Market by Target Indication
20.4.1.1. Human Microbiome Diagnostic Tests Market for Inflammatory Bowel Syndrome, Till 2035
20.4.1.2. Human Microbiome Diagnostic Tests Market for Irritable Bowel Disease, Till 2035
20.4.1.3. Human Microbiome Diagnostic Tests Market for Colorectal Cancer, Till 2035
20.4.1.4. Human Microbiome Diagnostic Tests Market for Diabetes Mellitus, Till 2035
20.4.2. Human Microbiome Diagnostic Tests Market: Distribution by Therapeutic Area
20.4.2.1. Human Microbiome Diagnostic Tests Market for Digestive and Gastrointestinal Disorders, Till 2035
20.4.2.2. Human Microbiome Diagnostic Tests Market for Oncological Disorders, Till 2035
20.4.2.3. Human Microbiome Diagnostic Tests Market for Metabolic Disorders, Till 2035
20.4.3. Human Microbiome Diagnostic Tests Market: Distribution by Key Geographical Regions
20.4.3.1. Human Microbiome Diagnostic Tests Market in North America, Till 2035
20.4.3.2. Human Microbiome Diagnostic Tests Market in Europe, Till 2035
20.4.3.3. Human Microbiome Diagnostic Tests Market in Asia Pacific, Till 2035
21. FECAL MICROBIOTA THERAPIES: MARKET FORECAST AND OPPORTUNITY ANALYSIS
21.1. Chapter Overview
21.2. Forecast Methodology and Key Assumptions
21.3. Global Fecal Microbiota Therapies Market, Till 2035
21.3.1. Global Fecal Microbiota Therapies Market, Till 2035 (By Value)
21.3.2. Global Fecal Microbiota Therapies Market, Till 2035 (By Value)
21.3.3. Overall FMT Market, Till 2035 (By Volume)
21.4. Overall Microbiome Market by Product Offerings, Till 2035
22. CASE STUDY: MICROBIOME-BASED PRODUCTS IN NON-PHARMACEUTICAL INDUSTRIES
22.1. Chapter Overview
22.2. List of Microbiome Products in Non-Pharmaceutical Industry
22.2.1. Applications of Microbiome Based Products in Cosmetics and Food Industry
22.3. Applications of Microbiome Based Products in Agriculture Industry
22.4. Future Perspectives
LIST OF FIGURES
Figure 2.1 Executive Summary: Overall Market Landscape of Human Microbiome Therapeutics
Figure 2.2 Executive Summary: Overall Market Landscape of Human Microbiome Diagnostics and Screening / Profiling Tests
Figure 2.3 Executive Summary: Overall Market Landscape of Fecal Microbiota Therapies and Clinical Trial Analysis
Figure 2.4 Executive Summary: Partnerships and Collaborations
Figure 2.5 Executive Summary: Funding and Investment Analysis
Figure 2.6 Executive Summary: Market Sizing and Opportunity Analysis for Microbiome Therapeutics
Figure 2.7 Executive Summary: Market Sizing and Opportunity Analysis for Microbiome Diagnostics and Fecal Microbiota Therapies
Figure 3.1 Benefits of the Human Microbiota
Figure 3.2 Factors Affecting Gut Microbiota
Figure 3.3 Factors Affecting Gut Microbiota in Infants
Figure 3.4 Impact of Diet on Gut Microbiota
Figure 3.5 Types of Microbiome-Based Therapeutics
Figure 3.6 Approaches to Design Microbiome Therapeutics
Figure 3.7 Health Benefits of Probiotics
Figure 3.8 Mechanism of Action of Probiotics
Figure 3.9 Beneficial Bacterial Strains for Probiotics
Figure 3.10 Key Achievements of the Human Microbiome Project (HMP)
Figure 3.11 Challenges Associated with the Development of Microbiome Therapeutics
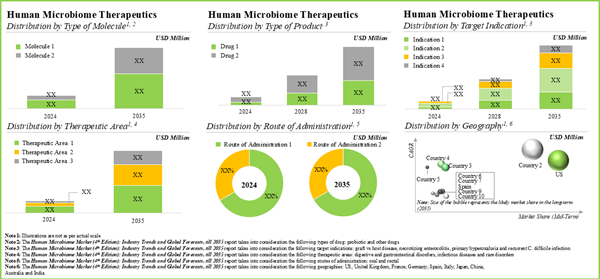
Figure 4.1 Clinical Phase Microbiome Therapeutics: Distribution by Phase of Development
Figure 4.2 Clinical Phase Microbiome Therapeutics: Distribution by Type of Molecule
Figure 4.3. Clinical Phase Microbiome Therapeutics: Distribution by Phase of Development and Type of Molecule
Figure 4.4 Clinical Phase Microbiome Therapeutics: Distribution by Type of Biologic
Figure 4.5 Clinical Phase Microbiome Therapeutics: Distribution by Type of Product
Figure 4.6 Clinical Phase Microbiome Therapeutics: Distribution by Target Indication
Figure 4.7 Clinical Phase Microbiome Therapeutics: Distribution by Therapeutic Area
Figure 4.8 Clinical Phase Microbiome Therapeutics: Distribution by Route of Administration
Figure 4.9 Clinical Phase Microbiome Therapeutics: Distribution by Type of Formulation
Figure 4.10 Clinical Phase Microbiome Therapeutics: Distribution by Dose Frequency
Figure 4.11 Clinical Phase Microbiome Therapeutics: Distribution by Type of Therapy
Figure 4.12 Clinical Phase Microbiome Therapeutics: Distribution by Combination Drug
Figure 4.13 Preclinical Phase Microbiome Therapeutics: Distribution by Phase of Development
Figure 4.14 Preclinical Phase Microbiome Therapeutics: Distribution by Type of Molecule
Figure 4.15 Preclinical Phase Microbiome Therapeutics: Distribution by Phase of Development and Type of Molecule
Figure 4.16 Preclinical Phase Microbiome Therapeutics: Distribution by Type of Biologic
Figure 4.17 Preclinical Phase Microbiome Therapeutics: Distribution by Type of Product
Figure 4.18 Preclinical Phase Microbiome Therapeutics: Distribution by Target Indication
Figure 4.19 Preclinical Phase Microbiome Therapeutics: Distribution by Therapeutic Area
Figure 4.20 Preclinical Phase Microbiome Therapeutics: Distribution by Route of Administration
Figure 4.21 Microbiome Therapeutic Developers: Distribution by Year of Establishment
Figure 4.22 Microbiome Therapeutic Developers: Distribution by Company Size
Figure 4.23 Microbiome Therapeutic Developers: Distribution by Location of Headquarters
Figure 4.24 Microbiome Therapeutic Developers: Distribution by Company Size and Location of Headquarters
Figure 5.1 Rebiotix: Financial Information, Since 2017 (USD Million)
Figure 5.2 Seres Therapeutics: Financial Information, Since 2017 (USD Million)
Figure 5.3 4D Pharma: Financial Information, Since 2017 (USD Million)
Figure 6.1 Key Steps in 16S rRNA Gene Sequence Analysis
Figure 6.2 Microbiome Diagnostics and Screening / Profiling Tests: Distribution by Stage of Development
Figure 6.3 Microbiome Diagnostics and Screening / Profiling Tests: Distribution by Type of Test
Figure 6.4 Microbiome Diagnostics and Screening / Profiling Tests: Distribution by Stage of Development and Type of Test
Figure 6.5 Microbiome Diagnostics and Screening / Profiling Tests: Distribution by Type of Sample Analyzed
Figure 6.6 Microbiome Diagnostics and Screening / Profiling Tests: Distribution by Type of Screening Technique
Figure 6.7 Microbiome Diagnostics and Screening / Profiling Tests: Distribution by Target Indication
Figure 6.8 Microbiome Diagnostics and Screening / Profiling Tests: Distribution by Therapeutic Area
Figure 6.9 Microbiome Diagnostics and Screening / Profiling Tests: Distribution by Purpose of Test
Figure 6.10 Microbiome Diagnostic and Screening / Profiling Tests Providers: Distribution by Year of Establishment
Figure 6.11 Microbiome Diagnostic and Screening / Profiling Tests Providers: Distribution by Company Size
Figure 6.12 Microbiome Diagnostic and Screening / Profiling Tests Providers: Distribution by Location of Headquarters
Figure 6.13 Microbiome Diagnostic and Screening / Profiling Tests Providers: Distribution by Company Size and Location of Headquarters
Figure 8.1 Working Mechanism of a Microflora Refinement System
Figure 8.2 Fecal Microbiota Therapies: Distribution by Application Area
Figure 8.3 Fecal Microbiota Therapies: Distribution by Status of Development
Figure 8.4 Fecal Microbiota Therapies: Distribution by Target Indication
Figure 8.5 Fecal Microbiota Therapies: Distribution by Therapeutic Area
Figure 8.6 Fecal Microbiota Therapies: Distribution by Route of Administration
Figure 8.7 Fecal Microbiota Therapies Developers: Distribution by Year of Establishment
Figure 8.8 Fecal Microbiota Therapies Developers: Distribution by Company Size
Figure 8.9 Fecal Microbiota Therapies Developers: Distribution by Location of Headquarters
Figure 9.1 Clinical Trial Analysis: Scope and Methodology
Figure 9.2 Clinical Trial Analysis: Distribution by Trial Status
Figure 9.3 Clinical Trial Analysis: Cumulative Distribution by Trial Registration Year, Since Pre 2015
Figure 9.4 Clinical Trial Analysis: Distribution by Trial Registration Year and Enrolled Patient Population, Since Pre 2015
Figure 9.5 Clinical Trial Analysis: Distribution by Trial Registration Year and Trial Recruitment Status
Figure 9.6 Clinical Trial Analysis: Distribution by Trial Phase and Number of Patients Enrolled
Figure 9.7 Clinical Trial Analysis: Distribution by Study Design
Figure 9.8 Leading Industry Players: Distribution by Number of Registered Trials
Figure 9.9 Leading Non-industry Players: Distribution by Number of Registered Trials\
Figure 9.10 Clinical Trial Analysis: Analysis by Trial Location
Figure 9.11 Clinical Trial Analysis: Analysis by Trial Status and Geography
Figure 10.1 AC Matrix: Pictorial Representation
Figure 10.2 AC Matrix: Positioning of Different Indications
Figure 11.1 Benchmarking of Start-ups: Distribution by Portfolio Diversity
Figure 11.2 Benchmarking of Start-ups: Distribution by Phase of Development
Figure 11.3 Benchmarking of Start-ups: Distribution by Indication Diversity
Figure 11.4 Benchmarking of Start-ups: Distribution by Funding Amount
Figure 11.5 Benchmarking of Start-ups: Distribution by Partnership Activity
Figure 11.6 Start-up Health Indexing: Roots Analysis Perspective
Figure 11.7 Start-up Heal Indexing: Leading Companies
Figure 12.1 Diabetes: Diagnostic Limits for Plasma Glucose Levels
Figure 12.2 Non-Insulin Therapies for Diabetes
Figure 12.3 Weight Categories as per BMI Calculations
Figure 12.4 Causes of Obesity in Children
Figure 12.5 Obese Population: Distribution by Key Regions
Figure 13.1 Partnerships and Collaborations: Distribution of Cumulative Year-wise Trend, Since 2017
Figure 13.2 Partnerships and Collaborations: Distribution of Type of Partnership
Figure 13.3 Partnerships and Collaborations: Distribution of Year and Type of Partnership, Since 2017
Figure 13.4 Partnerships and Collaborations: Distribution of Type of Collaborator
Figure 13.5 Partnerships and Collaborations: Distribution of Target Indication
Figure 13.6 Partnerships and Collaborations: Distribution by Type of Partnership and Target Indication
Figure 13.7 Partnerships and Collaborations: Distribution of Therapeutic Area
Figure 13.8 Most Active Players: Distribution by Number of Partnerships
Figure 13.9 Partnerships and Collaborations: Distribution by Type of Agreement (Country wise)
Figure 13.10 Partnerships and Collaborations: Distribution by Type of Agreement (Region wise)
Figure 13.11 Partnerships and Collaborations: Intercontinental and Intracontinental Agreements
Figure 14.1 Funding and Investment Analysis: Distribution by Year, Type of Funding and Amount Invested (USD Million), Since 2017
Figure 14.2 Funding and Investment Analysis: Distribution by Year of Establishment and Type of Funding, Since 2017
Figure 14.3 Funding and Investment Analysis: Cumulative Year-wise Distribution of Funding Instances, Since 2017
Figure 14.4 Funding and Investment Analysis: Cumulative Year-wise Distribution of Amount Invested (USD Million), Since 2017
Figure 14.5 Funding and Investment Analysis: Distribution by Type of Funding
Figure 14.6 Funding and Investment Analysis: Distribution by Year and Type of Funding
Figure 14.7 Funding and Investment Analysis: Distribution by Type of Funding and Amount Invested (USD Million)
Figure 14.8 Funding and Investment Analysis: Distribution by Purpose of Funding
Figure 14.9 Funding and Investment Analysis: Distribution by Type of Product
Figure 14.10 Funding and Investment Analysis: Distribution by Target Indication
Figure 14.11 Funding and Investment Analysis: Distribution by Therapeutic Area
Figure 14.12 Funding and Investment Analysis: Distribution by Type of Product, Type of Funding and Amount Invested (USD Million)
Figure 14.13 Funding and Investment Analysis: Distribution by Region
Figure 14.14 Most Active Players: Distribution by Number of Instances
Figure 14.15 Most Active Players: Distribution by Amount Raised (USD Million)
Figure 14.16 Key Investors: Distribution by Number of Funding Instances
Figure 14.17 Funding and Investment Summary, Since 2017 (USD Million)
Figure 15.1 Live Biotherapeutic Products and Microbiome Contract Manufacturers: Distribution by Year of Establishment
Figure 15.2 Live Biotherapeutic Products and Microbiome Contract Manufacturers: Distribution by Company Size
Figure 15.3 Live Biotherapeutic Products and Microbiome Contract Manufacturers: Distribution by Location of Headquarters
Figure 15.4 Live Biotherapeutic Products and Microbiome Contract Manufacturers: Distribution by Scale of Operation
Figure 15.5 Live Biotherapeutic Products and Microbiome Contract Manufacturers: Distribution by Type of Product Manufactured
Figure 15.6 Live Biotherapeutic Products and Microbiome Contract Manufacturers: Distribution by Type of Formulation
Figure 15.7 Live Biotherapeutic Products and Microbiome Contract Manufacturers: Distribution by Scale of Operation and Type of Formulation
Figure 16.1 Big Data: The Three V’s
Figure 16.2 Internet of Things: Framework
Figure 16.3 Internet of Things: Applications in Healthcare
Figure 16.4 Big Data: Google Trends Analysis
Figure 16.5 Big Data and Microbiome: Google Trends
Figure 16.6 Big Data: Key Application Areas
Figure 16.7 Big Data: Opportunities in Healthcare
Figure 16.8 Big Data: Key Benefits for Pharmaceutical and Biotechnology Products
Figure 16.9 Challenges Associated with Microbiome-related Big Data Management
Figure 17.1 Global Human Microbiome Therapeutics Market, Till 2035 (USD Billion)
Figure 17.2 Human Microbiome Therapeutics Market: Distribution by Type of Product (USD Million)
Figure 17.3 Human Microbiome Therapeutics Market for Probiotic Drugs, Till 2035 (USD Million)
Figure 17.4 Human Microbiome Therapeutics Market for Other Drugs, Till 2035 (USD Million)
Figure 17.5 Human Microbiome Therapeutics Market: Distribution by Target Disease Indication
Figure 17.6 Human Microbiome Therapeutics Market for Recurrent C. difficile Infection, Till 2035 (USD Million)
Figure 17.7 Human Microbiome Therapeutics Market for Necrotizing Enterocolitis, Till 2035 (USD Million)
Figure 17.8 Human Microbiome Therapeutics Market for Primary Hyperoxaluria, Till 2035 (USD Million)
Figure 17.9 Human Microbiome Therapeutics Market for Graft versus Host Disease, Till 2035 (USD Million)
Figure 17.10 Human Microbiome Therapeutics Market: Distribution by Therapeutic Area (USD Million)
Figure 17.11 Human Microbiome Therapeutics Market for Infectious Diseases, Till 2035 (USD Million)
Figure 17.12 Human Microbiome Therapeutics Market for Digestive and Gastrointestinal Disorders, Till 2035 (USD Million)
Figure 17.13 Human Microbiome Therapeutics Market for Rare Disorders, Till 2035 (USD Million)
Figure 17.14 Human Microbiome Therapeutics Market: Distribution by Route of Administration (USD Million)
Figure 17.15 Human Microbiome Therapeutics Market for Oral Therapeutics, Till 2035 (USD Million)
Figure 17.16 Human Microbiome Therapeutics Market for Rectal Therapeutics, Till 2035 (USD Million)
Figure 17.17 Human Microbiome Therapeutics Market: Distribution by Type of Formulation (USD Million)
Figure 17.18 Human Microbiome Therapeutics Market for Capsules, Till 2035 (USD Million)
Figure 17.19 Human Microbiome Therapeutics Market for Suspensions, Till 2035 (USD Million)
Figure 17.20 Human Microbiome Therapeutics Market for Enemas, Till 2035 (USD Million)
Figure 17.21 Human Microbiome Therapeutics Market: Distribution by Key Geographical Regions, Till 2035 (USD Million)
Figure 17.22 Human Microbiome Therapeutics Market in North America, Till 2035 (USD Million)
Figure 17.23 Human Microbiome Therapeutics Market in Europe, Till 2035 (USD Million)
Figure 17.24 Human Microbiome Therapeutics Market in Asia-Pacific and Rest of the World, Till 2035 (USD Million)
Figure 17.25 Human Microbiome Therapeutics Market: Distribution by Leading Drug Developers, Till 2035 (USD Million)
Figure 17.26 Sales Forecast: SER-109, Till 2035 (USD Million)
Figure 17.27 Sales Forecast: RBX2660, Till 2035 (USD Million)
Figure 17.28 Sales Forecast: CP101, Till 2035 (USD Million)
Figure 17.29 Sales Forecast: IBP-9414, Till 2035 (USD Million)
Figure 17.30 Sales Forecast: Oxabact, Till 2035 (USD Million)
Figure 17.31 Sales Forecast: MaaT013, Till 2035 (USD Million)
Figure 18.1 Overall Microbiome Diagnostics Market, Till 2035 (USD Million)
Figure 18.2 Microbiome Diagnostics Market: Distribution by Target Indications, Till 2035 (USD Million)
Figure 18.3 Microbiome Diagnostics Market for Irritable Bowel Syndrome, Till 2035 (USD Million)
Figure 18.4 Microbiome Diagnostics Market for Inflammatory Bowel Disease, Till 2035 (USD Million)
Figure 18.5 Microbiome Diagnostics Market for Colorectal Cancer, Till 2035 (USD Million)
Figure 18.6 Microbiome Diagnostics Market for Diabetes Mellitus, Till 2035 (USD Million)
Figure 18.7 Microbiome Diagnostics Market: Distribution by Therapeutic Areas, Till 2035 (USD Million)
Figure 18.8 Microbiome Diagnostics Market for Digestive and Gastrointestinal Disorders, Till 2035 (USD Million)
Figure 18.9 Microbiome Diagnostics Market for Oncological Disorders, Till 2035 (USD Million)
Figure 18.10 Microbiome Diagnostics Market for Metabolic Disorders, Till 2035 (USD Million)
Figure 18.11 Microbiome Diagnostics Market: Distribution by Key Geographical Regions, Till 2035 (USD Million)
Figure 18.12 Microbiome Diagnostics Market in North America, Till 2035 (USD Million)
Figure 18.13 Microbiome Diagnostics Market in Europe, Till 2035 (USD Million)
Figure 18.14 Microbiome Diagnostics Market in Asia-Pacific and Rest of the World, Till 2035 (USD Million)
Figure 19.1 Global Fecal Microbiota Therapies Market, Till 2035 (USD Million)
Figure 19.2 Global Microbiota Therapies Market, Till 2035 (Million Procedures)
Figure 19.3 Fecal Microbiota Therapies Market in North America, Till 2035 (USD Million)
Figure 19.4 Fecal Microbiota Therapies Market in Europe, Till 2035 (USD Million)
Figure 19.5 Fecal Microbiota Therapies Market in Asia Pacific, Till 2035 (USD Million)
Figure 19.6 Fecal Microbiota Therapies Market in North America, Till 2035 (Million Procedures)
Figure 19.7 Fecal Microbiota Therapies in Europe, Till 2035 (Million Procedures)
Figure 19.8 Fecal Microbiota Therapies Market in Asia Pacific, Till 2035 (Million Procedures)
Figure 19.9 Overall Human Microbiome Market: Distribution by Product Offerings (USD Million)
Figure 21.1 Concluding Remarks: Human Microbiome Therapeutics Landscape
Figure 21.2 Concluding Remarks: Human Microbiome Therapeutics Landscape
Figure 21.3 Concluding Remarks: Human Microbiome Diagnostics Landscape
Figure 21.4 Concluding Remarks: Fecal Microbiota Therapies Landscape
Figure 21.5 Concluding Remarks: Funding and Investment Analysis
Figure 21.6 Concluding Remarks: Partnerships and Collaborations
Figure 21.7 Concluding Remarks: Start-up Health Indexing
Figure 21.8 Concluding Remarks: Human Microbiome Market Sizing and Opportunity Analysis
Figure 21.9 Concluding Remarks: Microbiome Therapeutics Market Sizing and Opportunity Analysis
Figure 21.10 Concluding Remarks: Microbiome Diagnostic Tests Market Sizing and Opportunity Analysis
LIST OF TABLES
Table 3.1 Types of Microbiota in the Gastrointestinal Tract
Table 3.2 Common Instances of Misuse of Antibiotics
Table 3.3 Impact of Antibiotics on Intestinal Microflora
Table 3.4 List of Microorganisms Classified as Class I Carcinogens by the IARC
Table 3.5 Relationship Between Microbiome and Disease Progression
Table 3.6 Impact of Drug-Microbiome Interactions
Table 3.7 List of Foods Containing Prebiotics
Table 4.1 Microbiome Therapeutics: Clinical Pipeline
Table 4.2 Clinical Phase Microbiome Therapeutics: Information on Type of Molecule and Route of Administration
Table 4.3 Clinical Phase Microbiome Therapeutics: Additional Information
Table 4.4 Microbiome Therapeutics: Preclinical Pipeline
Table 4.5 Preclinical Phase Microbiome Therapeutics: Additional Information
Table 4.6 Microbiome Therapeutics: List of Drug Developers
Table 5.1 Microbiome Therapeutic Developers: Companies with Candidates in Highest Phase of Development
Table 5.2 Finch Therapeutics: Microbiome-Based Product Portfolio
Table 5.3 CP101: Current Status of Development
Table 5.4 CP101: Clinical Studies
Table 5.5 Finch Therapeutics: Recent Developments and Future Outlook
Table 5.6 Infant Bacterial Therapeutics: Microbiome-Based Product Portfolio
Table 5.7 IBP-9414: Current Status of Development
Table 5.8 IBP-9414: Clinical Studies
Table 5.9 Infant Bacterial Therapeutics: Recent Developments and Future Outlook
Table 5.10 MaaT Pharma: Microbiome-Based Product Portfolio
Table 5.11 MaaT013: Current Status of Development
Table 5.12 MaaT013: Clinical Studies
Table 5.13 MaaT Pharma: Recent Developments and Future Outlook
Table 5.14 OxThera: Microbiome-Based Product Portfolio
Table 5.15 Oxabact: Current Status of Development
Table 5.16 Oxabact: Clinical Studies
Table 5.17 OxThera: Recent Developments and Future Outlook
Table 5.18 Rebiotix: Microbiome-Based Product Portfolio
Table 5.19 RBX2660: Current Status of Development
Table 5.20 RBX2660: Clinical Studies
Table 5.21 Rebiotix: Recent Developments and Future Outlook
Table 5.22 Seres Therapeutics: Microbiome-Based Product Portfolio
Table 5.23 SER-109: Current Status of Development
Table 5.24 SER-109: Clinical Studies
Table 5.25 Seres Therapeutics: Recent Developments and Future Outlook
Table 5.26 Microbiome Therapeutics Developers: Companies with Maximum Number of Therapeutic Programs
Table 5.27 4D Pharma: Financial Information
Table 5.28 4D Pharma: Recent Developments and Future Outlook
Table 5.29 Biosortia Pharmaceuticals: Recent Developments and Future Outlook
Table 5.30 Qu Biologics: Recent Developments and Future Outlook
Table 5.31 Servatus: Recent Developments and Future Outlook
Table 6.1 Microbiome Diagnostics and Screening / Profiling Tests: Marketed and Development Pipeline
Table 6.2 Microbiome Diagnostics and Screening / Profiling Tests: List of Developers
Table 7.1 Shoreline Biome: Company Overview
Table 7.2 Shoreline Biome: Microbiome Test Portfolio
Table 7.3 Shoreline Biome: Recent Developments and Future Outlook
Table 7.4 DNA Genotek: Company Overview
Table 7.5 DNA Genotek: Microbiome Test Portfolio
Table 7.6 DNA Genotek: Recent Developments and Future Outlook
Table 7.7 Invivo Healthcare: Company Overview
Table 7.8 Invivo Healthcare: Microbiome Test Portfolio
Table 7.9 Invivo Healthcare: Recent Developments and Future Outlook
Table 7.10 GoodGut: Company Overview
Table 7.11 GoodGut: Microbiome Test Portfolio
Table 7.12 GoodGut: Recent Developments and Future Outlook
Table 7.13 BiomeDx: Company Overview
Table 7.14 BiomeDx: Microbiome Test Portfolio
Table 7.15 BiomeDx: Recent Developments and Future Outlook
Table 8.1 Comparison between Various Routes of Administration of FMT
Table 8.2 FMT: Summary of Clinical Guidelines
Table 8.3 FMT: Summary of Insurance Coverage Payer
Table 8.4 FMT: Marketed and Development Pipeline
Table 8.5 FMT: List of Developers
Table 8.6 List of Stool Banks for FMT Development
Table 8.7 Fecal Microbiota Transplant and Human Microbiome Transplant (HMT): Key Differences in Processing
Table 8.8 Flora Medicine: Fecal Microbiota Transplant Treatment Cost
Table 8.9 OpenBiome: Types of FMT Formulations
Table 9.1 Fecal Microbiota Transplant: List of Registered Clinical Trials
Table 12.1 Diabetes: Current Treatment Options
Table 12.2 Diabetes: Side Effects of Current Treatment Options
Table 12.3 Microbiome Therapeutics Candidates for Diabetes
Table 12.4 Microbiome Therapeutics Candidates for Lactose Intolerance
Table 12.5 Microbiome Therapeutics Candidates for NASH
Table 12.6 Microbiome Therapeutics Candidates for Primary Hyperoxaluria
Table 12.7 Obesity: Side Effects of Current Treatment Options
Table 12.8 Microbiome Therapeutic Candidates for Obesity
Table 12.9 Crohn’s Disease: Current Treatment Options
Table 12.10 Crohn’s Disease: Side Effects of Current Treatment Options
Table 12.11 Microbiome Therapeutics Candidates for Crohn’s Disease
Table 12.12 IBS: Current Treatment Options
Table 12.13 Microbiome Therapeutics Candidates for IBS
Table 12.14 Ulcerative Colitis: Current Treatment Options
Table 12.15 Ulcerative Colitis: Side Effects of Current Treatment Options
Table 12.16 Microbiome Therapeutics Candidates for Ulcerative Colitis
Table 12.17 Colorectal Cancer: Side Effects of Current Treatment Options
Table 12.18 Microbiome Therapeutics Pipeline for Colorectal Cancer
Table 12.19 Lung Cancer: Current Treatment Options
Table 12.20 Lung Cancer: Side Effects of Current Treatment Options
Table 12.21 Microbiome Therapeutics Candidates for Lung Cancer
Table 12.22 Acne Vulgaris: Current Treatment Options
Table 12.23 Acne Vulgaris: Side Effects of Current Treatment Options
Table 12.24 Microbiome Therapeutics Candidates for Acne Vulgaris
Table 12.25 CDI: Diagnostic Testing
Table 12.26 CDI: Severity Scoring System and Treatment Options
Table 12.27 CDI: Side Effects of Current Treatment Options
Table 12.28 Microbiome Therapeutics Candidates for CDI
Table 12.29 Bacterial Vaginosis: Current Treatment Options
Table 12.30 Bacterial Vaginosis: Side Effects of Current Treatment Options
Table 12.31 Microbiome Therapeutics Candidates for Bacterial Vaginosis
Table 13.1 Human Microbiome: List of Partnerships and Collaborations, Since 2017
Table 14.1 Microbiome Therapeutics and Diagnostics: Funding and Investments, Information on Funding Type, Year, Amount and Investor, Since 2017
Table 14.2 Microbiome Therapeutics and Diagnostics: Funding and Investments, Information on Type of Product, Target Indication and Focus Area, Since 2017
Table 14.3 Microbiome Therapeutics and Diagnostics: Funding and Investments, Information on Type of Investor and Location of Headquarters, Since 2017
Table 15.1 Microbiome Contract Manufacturers: Information on Year of Establishment, Headquarters, Company Size, Accreditation Received and Scale of Operation
Table 15.2 Microbiome Contract Manufacturers: Information on Type of Product Manufactured
Table 15.3 Microbiome Contract Manufacturers: Information on Type of Formulation
Table 15.4 Comparison of Key Factors for the Selection of Contract Service Providers: Harvey Ball Analysis
Table 16.1 List of Companies Using Big Data for Microbiome Research
Table 16.2 Human Longevity: Partnerships and Collaborations
Table 16.3 Human Longevity: Venture Capital Funding
Table 16.4 Resilient Biotics: Venture Capital Funding
Table 20.1 List of Companies Engaged in the Development of Microbiome Products for Other Applications
Table 20.2 Pipeline of Microbiome Based Consumer Products, Medical Foods and Supplements
Table 22.1 Quorum Innovations: Company / Organization Snapshot
Table 22.2 Floragraph: Company / Organization Snapshot
Table 22.3 Pacific Northwest National Laboratories: Company / Organization Snapshot
Table 22.4 Chung Mei Pharmaceutical: Company / Organization Snapshot
Table 22.5 Universal Stabilization technologies: Company / Organization Snapshot
Table 22.6 BiomX: Company / Organization Snapshot
Table 22.7 List Biological Laboratories: Company / Organization Snapshot
Table 22.8 S-Biomedic: Company / Organization Snapshot
Table 22.9 Pendulum Therapeutics: Company / Organization Snapshot
Table 22.10 Siolta Therapeutics: Company / Organization Snapshot
Table 22.11 OpenBiome: Company / Organization Snapshot
Table 22.12 Metabiomics: Company / Organization Snapshot
Table 22.13 Assembly Biosciences: Company / Organization Snapshot
Table 22.14 Microbiome Therapeutics: Company / Organization Snapshot
Table 22.15 Da Volterra: Company Snapshot
Table 23.1 Clinical Phase Microbiome Therapeutics: Distribution by Phase of Development
Table 23.2 Clinical Phase Microbiome Therapeutics: Distribution by Type of Molecule
Table 23.3 Clinical Phase Microbiome Therapeutics: Distribution by Phase of Development and Type of Molecule
Table 23.4 Clinical Phase Microbiome Therapeutics: Distribution by Type of Biologic
Table 23.5 Clinical Phase Microbiome Therapeutics: Distribution by Type of Product
Table 23.6 Clinical Phase Microbiome Therapeutics: Distribution by Target Indication
Table 23.7 Clinical Phase Microbiome Therapeutics: Distribution by Therapeutic Area
Table 23.8 Clinical Phase Microbiome Therapeutics: Distribution by Route of Administration
Table 23.9 Clinical Phase Microbiome Therapeutics: Distribution by Type of Formulation
Table 23.10 Clinical Phase Microbiome Therapeutics: Distribution by Dose Frequency
Table 23.11 Clinical Phase Microbiome Therapeutics: Distribution by Type of Therapy
Table 23.12 Clinical Phase Microbiome Therapeutics: Distribution by Combination Drug
Table 23.13 Preclinical Phase Microbiome Therapeutics: Distribution by Phase of Development
Table 23.14 Preclinical Phase Microbiome Therapeutics: Distribution by Type of Molecule
Table 23.15 Preclinical Phase Microbiome Therapeutics: Distribution by Phase of Development and Type of Molecule
Table 23.16 Preclinical Phase Microbiome Therapeutics: Distribution by Type of Biologic
Table 23.17 Preclinical Phase Microbiome Therapeutics: Distribution by Type of Product
Table 23.18 Preclinical Phase Microbiome Therapeutics: Distribution by Target Indication
Table 23.19 Preclinical Phase Microbiome Therapeutics: Distribution by Therapeutic Area
Table 23.20 Microbiome Therapeutic Developers: Distribution by Year of Establishment
Table 23.21 Microbiome Therapeutic Developers: Distribution by Company Size
Table 23.22 Microbiome Therapeutic Developers: Distribution by Location of Headquarters
Table 23.23 Seres Therapeutics: Financial Information, Since 2017 (USD Million)
Table 23.24 4D Pharma: Financial Information, Since 2017 (USD Million)
Table 23.25 Ferring Pharmaceuticals: Financial Information, Since 2019 (USD Million)
Table 23.26 Microbiome Diagnostics and Screening / Profiling Tests: Distribution by Stage of Development
Table 23.27 Microbiome Diagnostics and Screening / Profiling Tests: Distribution by Type of Test
Table 23.28 Microbiome Diagnostics and Screening / Profiling Tests: Distribution by Stage of Development and Type of Test
Table 23.29 Microbiome Diagnostics and Screening / Profiling Tests: Distribution by Type of Sample Analyzed
Table 23.30 Microbiome Diagnostics and Screening / Profiling Tests: Distribution by Type of Screening Technique
Table 23.31 Microbiome Diagnostics and Screening / Profiling Tests: Distribution by Target Indication
Table 23.32 Microbiome Diagnostics and Screening / Profiling Tests: Distribution by Therapeutic Area
Table 23.33 Microbiome Diagnostics and Screening / Profiling Tests: Distribution by Purpose of Test
Table 23.34 Microbiome Diagnostic and Screening / Profiling Tests Providers: Distribution by Year of Establishment
Table 23.35 Microbiome Diagnostic and Screening / Profiling Tests Providers: Distribution by Company Size
Table 23.36 Microbiome Diagnostic and Screening / Profiling Tests Providers: Distribution by Location of Headquarters
Table 23.37 Most Active Microbiome Test Providers: Distribution by Number of Microbiome Tests
Table 23.38 Fecal Microbiota Transplantation: Distribution by Application Area
Table 23.39 Fecal Microbiota Transplantation: Distribution by Status of Development
Table 23.40 Fecal Microbiota Transplantation: Distribution by Target Indication
Table 23.41 Fecal Microbiota Transplantation: Distribution by Therapeutic Area
Table 23.42 Fecal Microbiota Transplantation: Distribution by Route of Administration
Table 23.43 Fecal Microbiota Transplantation Developers: Distribution by Year of Establishment
Table 23.44 Fecal Microbiota Transplantation Developers: Distribution by Company Size
Table 23.45 Fecal Microbiota Transplantation Developers: Distribution by Location of Headquarters
Table 23.46 Clinical Trial Analysis: Distribution by Trial Status
Table 23.47 Clinical Trial Analysis: Cumulative Distribution by Trial Registration Year, Since Pre 2015
Table 23.48 Clinical Trial Analysis: Distribution by Trial Registration Year and Enrolled Patient Population, Since Pre 2015
Table 23.49 Clinical Trial Analysis: Distribution by Trial Registration Year and Trial Recruitment Status
Table 23.50 Clinical Trial Analysis: Distribution by Trial Phase and Number of Patients Enrolled
Table 23.51 Clinical Trial Analysis: Distribution by Study Design
Table 23.52 Leading Industry Players: Distribution by Number of Registered Trials
Table 23.53 Leading Non-industry Players: Distribution by Number of Registered Trials\
Table 23.54 Clinical Trial Analysis: Analysis by Trial Location
Table 23.55 Clinical Trial Analysis: Analysis by Trial Status and Geography
Table 23.56 Benchmarking of Start-ups: Distribution by Portfolio Diversity
Table 23.57 Benchmarking of Start-ups: Distribution by Phase of Development
Table 23.58 Benchmarking of Start-ups: Distribution by Indication Diversity
Table 23.59 Benchmarking of Start-ups: Distribution by Funding Amount
Table 23.60 Benchmarking of Start-ups: Distribution by Partnership Activity
Table 23.61 Partnerships and Collaborations: Distribution of Cumulative Year-wise Trend, Since 2017
Table 23.62 Partnerships and Collaborations: Distribution of Type of Partnership
Table 23.63 Partnerships and Collaborations: Distribution of Year and Type of Partnership, Since 2017
Table 23.64 Partnerships and Collaborations: Distribution of Type of Collaborator
Table 23.65 Partnerships and Collaborations: Distribution of Target Indication
Table 23.66 Partnerships and Collaborations: Distribution by Type of Partnership and Target Indication
Table 23.67 Partnerships and Collaborations: Distribution of Therapeutic Area
Table 23.68 Most Active Players: Distribution by Number of Partnerships
Table 23.69 Partnerships and Collaborations: Distribution by Type of Agreement (Country wise)
Table 23.70 Partnerships and Collaborations: Distribution by Type of Agreement (Region wise)
Table 23.71 Partnerships and Collaborations: Intercontinental and Intracontinental Agreements
Table 23.72 Funding and Investment Analysis: Cumulative Year-wise Distribution of Funding Instances, Since 2017
Table 23.73 Funding and Investment Analysis: Cumulative Year-wise Distribution of Amount Invested (USD Million), Since 2017
Table 23.74 Funding and Investment Analysis: Distribution by Type of Funding
Table 23.75 Funding and Investment Analysis: Distribution by Year and Type of Funding
Table 23.76 Funding and Investment Analysis: Distribution by Type of Funding and Amount Invested (USD Million)
Table 23.77 Funding and Investment Analysis: Distribution by Purpose of Funding
Table 23.78 Funding and Investment Analysis: Distribution by Type of Product
Table 23.79 Funding and Investment Analysis: Distribution by Target Indication
Table 23.80 Funding and Investment Analysis: Distribution by Therapeutic Area
Table 23.81 Funding and Investment Analysis: Distribution by Type of Product, Type of Funding and Amount Invested (USD Million)
Table 23.82 Funding and Investment Analysis: Distribution by Region
Table 23.83 Most Active Players: Distribution by Number of Instances
Table 23.84 Most Active Players: Distribution by Amount Raised (USD Million)
Table 23.85 Key Investors: Distribution by Number of Funding Instances
Table 23.86 Live Biotherapeutic Products and Microbiome Contract Manufacturers: Distribution by Year of Establishment
Table 23.87 Live Biotherapeutic Products and Microbiome Contract Manufacturers: Distribution by Company Size
Table 23.88 Live Biotherapeutic Products and Microbiome Contract Manufacturers: Distribution by Location of Headquarters
Table 23.89 Live Biotherapeutic Products and Microbiome Contract Manufacturers: Distribution by Scale of Operation
Table 23.90 Live Biotherapeutic Products and Microbiome Contract Manufacturers: Distribution by Type of Product Manufactured
Table 23.91 Live Biotherapeutic Products and Microbiome Contract Manufacturers: Distribution by Type of Formulation
Table 23.92 Live Biotherapeutic Products and Microbiome Contract Manufacturers: Distribution by Scale of Operation and Type of Formulation
Table 23.93 Global Human Microbiome Therapeutics Market, Till 2035 (USD Billion)
Table 23.94 Human Microbiome Therapeutics Market: Distribution by Type of Product (USD Million)
Table 23.95 Human Microbiome Therapeutics Market for Probiotic Drugs, Till 2035 (USD Million)
Table 23.96 Human Microbiome Therapeutics Market for Other Drugs, Till 2035 (USD Million)
Table 23.97 Human Microbiome Therapeutics Market: Distribution by Target Disease Indication
Table 23.98 Human Microbiome Therapeutics Market for Recurrent C. difficile Infection, Till 2035 (USD Million)
Table 23.99 Human Microbiome Therapeutics Market for Necrotizing Enterocolitis, Till 2035 (USD Million)
Table 23.100 Human Microbiome Therapeutics Market for Primary Hyperoxaluria, Till 2035 (USD Million)
Table 23.101 Human Microbiome Therapeutics Market for Graft versus Host Disease, Till 2035 (USD Million)
Table 23.102 Human Microbiome Therapeutics Market: Distribution by Therapeutic Area (USD Million)
Table 23.103 Human Microbiome Therapeutics Market for Infectious Diseases, Till 2035 (USD Million)
Table 23.104 Human Microbiome Therapeutics Market for Digestive and Gastrointestinal Disorders, Till 2035 (USD Million)
Table 23.105 Human Microbiome Therapeutics Market for Rare Disorders, Till 2035 (USD Million)
Table 23.106 Human Microbiome Therapeutics Market: Distribution by Route of Administration (USD Million)
Table 23.107 Human Microbiome Therapeutics Market for Oral Therapeutics, Till 2035 (USD Million)
Table 23.108 Human Microbiome Therapeutics Market for Rectal Therapeutics, Till 2035 (USD Million)
Table 23.109 Human Microbiome Therapeutics Market: Distribution by Type of Formulation (USD Million)
Table 23.110 Human Microbiome Therapeutics Market for Capsules, Till 2035 (USD Million)
Table 23.111 Human Microbiome Therapeutics Market for Suspensions, Till 2035 (USD Million)
Table 23.112 Human Microbiome Therapeutics Market for Enemas, Till 2035 (USD Million)
Table 23.113 Human Microbiome Therapeutics Market: Distribution by Key Geographical Regions, Till 2035 (USD Million)
Table 23.114 Human Microbiome Therapeutics Market in North America, Till 2035 (USD Million)
Table 23.115 Human Microbiome Therapeutics Market in Europe, Till 2035 (USD Million)
Table 23.116 Human Microbiome Therapeutics Market in Asia-Pacific and Rest of the World, Till 2035 (USD Million)
Table 23.117 Human Microbiome Therapeutics Market: Distribution by Leading Drug Developers, Till 2035 (USD Million)
Table 23.118 Sales Forecast: SER-109, Till 2035 (USD Million)
Table 23.119 Sales Forecast: RBX2660, Till 2035 (USD Million)
Table 23.120 Sales Forecast: CP101, Till 2035 (USD Million)
Table 23.121 Sales Forecast: IBP-9414, Till 2035 (USD Million)
Table 23.122 Sales Forecast: Oxabact, Till 2035 (USD Million)
Table 23.123 Sales Forecast: MaaT013, Till 2035 (USD Million)
Table 23.124 Overall Microbiome Diagnostics Market, Till 2035 (USD Million)
Table 23.125 Microbiome Diagnostics Market: Distribution by Target Indications, Till 2035 (USD Million)
Table 23.126 Microbiome Diagnostics Market for Irritable Bowel Syndrome, Till 2035 (USD Million)
Table 23.127 Microbiome Diagnostics Market for Inflammatory Bowel Disease, Till 2035 (USD Million)
Table 23.128 Microbiome Diagnostics Market for Colorectal Cancer, Till 2035 (USD Million)
Table 23.129 Microbiome Diagnostics Market for Diabetes Mellitus, Till 2035 (USD Million)
Table 23.130 Microbiome Diagnostics Market: Distribution by Therapeutic Areas, Till 2035 (USD Million)
Table 23.131 Microbiome Diagnostics Market for Digestive and Gastrointestinal Disorders, Till 2035 (USD Million)
Table 23.132 Microbiome Diagnostics Market for Oncological Disorders, Till 2035 (USD Million)
Table 23.133 Microbiome Diagnostics Market for Metabolic Disorders, Till 2035 (USD Million)
Table 23.134 Microbiome Diagnostics Market: Distribution by Key Geographical Regions, Till 2035 (USD Million)
Table 23.135 Microbiome Diagnostics Market in North America, Till 2035 (USD Million)
Table 23.136 Microbiome Diagnostics Market in Europe, Till 2035 (USD Million)
Table 23.137 Microbiome Diagnostics Market in Asia-Pacific and Rest of the World, Till 2035 (USD Million)
Table 23.138 Global Fecal Microbiota Therapies Market, Till 2035 (USD Million)
Table 23.139 Global Microbiota Therapies Market, Till 2035 (Million Procedures)
Table 23.140 Fecal Microbiota Therapies Market in North America, Till 2035 (USD Million)
Table 23.141 Fecal Microbiota Therapies Market in Europe, Till 2035 (USD Million)
Table 23.142 Fecal Microbiota Therapies Market in Asia Pacific, Till 2035 (USD Million)
Table 23.143 Fecal Microbiota Therapies Market in North America, Till 2035 (Million Procedures)
Table 23.144 Fecal Microbiota Therapies in Europe, Till 2035 (Million Procedures)
Table 23.145 Fecal Microbiota Therapies Market in Asia Pacific, Till 2035 (Million Procedures)
Table 23.146 Overall Human Microbiome Market: Distribution by Product Offerings (USD Million)