1. PREFACE
1.1. Scope of the Report
1.2. Research Methodology
1.2.1. Research Assumptions
1.2.2. Project Methodology
1.2.3. Forecast Methodology
1.2.4. Robust Quality Control
1.2.5. Key Considerations
1.2.5.1. Demographics
1.2.5.2. Economic Factors
1.2.5.3. Government Regulations
1.2.5.4. Supply Chain
1.2.5.5. COVID Impact / Related Factors
1.2.5.6. Market Access
1.2.5.7. Healthcare Policies
1.2.5.8. Industry Consolidation
1.3 Key Questions Answered
1.4. Chapter Outlines
3. INTRODUCTION
3.1. Chapter Overview
3.2. Overview of Biopharmaceuticals
3.3. Manufacturing Biopharmaceuticals
3.3.1. Types of Expression Systems Used
3.3.1.1. Bacterial Expression Systems
3.3.1.2. Yeast Expression Systems
3.3.1.3. Insect Expression Systems
3.3.1.4. Plant Expression Systems
3.3.1.5. Mammalian Expression Systems
3.3.1.6. Fungal Expression Systems
3.3.2. Processing Steps
3.3.2.1. Upstream Processing
3.3.2.2. Downstream Processing
3.4. Overview of Contract Manufacturing
3.4.1. Contract Manufacturing Scenario in China
3.5. Need for Outsourcing in the Biopharmaceutical Industry
3.5.1. Biopharmaceutical Outsourcing in China: Regulatory Scenario
3.6. Commonly Outsourced Operations in the Biopharmaceutical Industry
3.7. Basic Guidelines for Selecting a CMO Partner
3.8. Advantages of Outsourcing Manufacturing Services
3.8.1. Benefits of Engaging Chinese Contract Service Providers
3.9. Risks and Challenges Associated with Biopharmaceutical Contract Manufacturing
3.9.1. Challenges Associated with Engaging Chinese Contract Service Providers
3.10. Future Perspective
4. CASE STUDY: COMPARISON OF SMALL MOLECULES AND LARGE MOLECULES
4.1. Chapter Overview
4.2. Small Molecule and Large Molecule Drugs / Therapies
4.2.1. Comparison of Key Characteristics
4.2.2. Comparison of Manufacturing Processes
4.2.3. Comparison of Key Manufacturing-Related Challenges
5. COMPETITIVE LANDSCAPE
5.1. Chapter Overview
5.2. Chinese Biopharmaceutical Contract Manufacturers: Overall Market Landscape
5.2.1. Analysis by Year of Establishment
5.2.2. Analysis by Company Size
5.2.3. Analysis by Scale of Operation
5.2.4. Analysis by Location of Headquarters
5.2.5. Analysis by Location of Manufacturing Facilities
5.2.6. Analysis by Type of Product
5.2.7. Analysis by Types of Services Offered
5.2.8. Analysis by Type of Biologic
5.2.9. Analysis by Expression System Used
5.2.10. Analysis by Type of Bioreactor Used
5.2.11. Analysis by Mode of Operation of Bioreactor
5.2.12. Analysis by Packaging Form Used
5.2.13. Analysis by Regulatory Accreditations / Certifications
6. COMPANY PROFILES
6.1. Chapter Overview
6.2. ChemPartner Biologics
6.2.1. Company Overview
6.2.2. Service Portfolio
6.2.2.1. Cell Line Development
6.2.2.2. Process Development
6.2.2.3. Formulation Development
6.2.2.4. Aseptic Filling and Freeze Drying
6.2.2.5. Drug Developability and Manuafcturing Study
6.2.2.6. Container Extractables and Leachables Study
6.2.2.7. Analytical Development and Testing
6.2.3. Manufacturing Facilities and Capabilities
6.2.4. Recent Developments and Future Outlook
6.3. JHL Biotech
6.3.1. Company Overview
6.3.2. Service Portfolio
6.3.2.1. Cell Line and Process Development
6.3.2.2. Analytical and Formulation Development
6.3.2.3. cGMP Manufacturing
6.3.2.4. Quality Systems
6.3.2.5. Regulatory Support
6.3.2.6. Project Management Services
6.3.3. Manufacturing Facilities and Capabilities
6.3.4. Recent Developments and Future Outlook
6.4. JOINN Biologics
6.4.1. Company Overview
6.4.2. Service Portfolio
6.4.2.1. Development Services
6.4.2.2. Manufacturing Services
6.4.2.3. Fill / Finish Services
6.4.2.4. Quality Assurance and Quality Control
6.4.3. Manufacturing Facilities and Capabilities
6.4.4. Recent Developments and Future Outlook
6.5 MabPlex
6.5.1. Company Overview
6.5.2. Service Portfolio
6.5.2.1. Development Services
6.5.2.2. GMP Manufacturing Services
6.5.3. Manufacturing Facilities and Capabilities
6.5.4. Recent Developments and Future Outlook
6.6. Mycenax Biotech
6.6.1. Company Overview
6.6.2. Service Portfolio
6.6.2.1. Cell Development Services
6.6.2.2. Process Development Services
6.6.2.3. Analytical and Quality Control
6.6.2.4. GMP Manuafacturing Services
6.6.3. Manufacturing Facilities and Capabilities
6.6.4. Recent Developments and Future Outlook
6.7. WuXi AppTec
6.7.1. Company Overview
6.7.2. Financial Information
6.7.3. Service Portfolio
6.7.3.1. Discovery Sciences
6.7.3.2. Development Services
6.7.3.3. Testing Services
6.7.3.4. Clinical Manufacturing Services
6.7.3.5. Commercial Manufacturing Services
6.7.3.6. Fill / Finish Operations
6.7.4. Manufacturing Facilities and Capabilities
6.7.5. Recent Developments and Future Outlook
7. PARTNERSHIPS
7.1. Chapter Overview
7.2. Partnership Models
7.3. Chinese Biopharmaceutical Contract Manufacturers: Recent Partnerships
7.3.1. Analysis by Year of Partnership
7.3.2. Analysis by Type of Partnership
7.3.3. Analysis by Scale of Operation
7.3.4. Analysis by Type of Biologic
7.3.5. Analysis by Focus Area
7.3.6. Analysis by Therapeutic Area
7.3.7. Most Active Players: Analysis by Number of Partnerships
7.3.8. Geographical Analysis
7.3.8.1. Geographical Distribution by Number of Partnerships
7.3.8.2. Intercontinental and Intracontinental Agreements
8. RECENT EXPANSIONS
8.1. Chapter Overview
8.2. Chinese Biopharmaceutical Contract Manufacturers: Recent Expansions
8.2.1. Analysis by Year of Expansion
8.2.2. Analysis by Type of Expansion
8.2.3. Analysis by Scale of Operation
8.2.4. Analysis by Type of Biologic
8.2.5. Analysis by Location of Expansion Project
8.2.6. Analysis by Capacity of Expanded Facility
8.2.7. Most Active Players: Analysis by Number of Expansions
8.2.8. Analysis by Region
9. CLINICAL TRIAL ANALYSIS
9.1. Chapter Overview
9.2. Scope and Methodology
9.3. Clinical Trial Analysis: Biologic Drugs
9.3.1. Analysis by Trial Registration Year
9.3.2. Analysis by Trial Phase
9.3.3. Analysis by Trial Status
9.3.4. Geographical Analysis by Number of Clinical Trials
9.3.5. Geographical Analysis by Enrolled Patient Population
9.3.6. Analysis of Enrolled Patient Population by Trial Registration Year
9.3.7. Analysis of Enrolled Patient Population by Trial Phase
9.3.8. Analysi by Type of Sponsor / Collaborator
9.3.9. Most Active Players: Analysis by Number of Registered Trials
9.3.10. Analysis by Clinical Trial Center
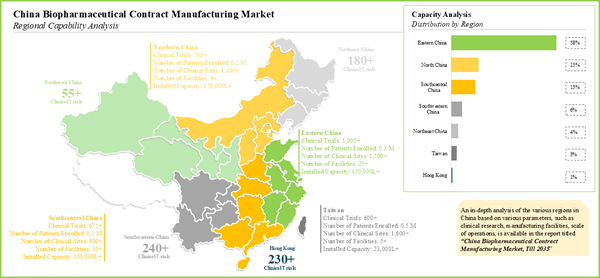
10. REGIONAL CAPABILITY ANALYSIS
10.1. Chapter Overview
10.2. Assumptions and Key Parameters
10.3. Overall Landscape of Chinese Biopharmaceuticals Contract Manufacturers
10.4. Regional Capability Analysis: Biopharmaceuticals Contract Manufacturers in Northern China
10.5. Regional Capability Analysis: Biopharmaceuticals Contract Manufacturers in Eastern China
10.6. Regional Capability Analysis: Biopharmaceuticals Contract Manufacturers in Central China
10.7. Regional Capability Analysis: Biopharmaceuticals Contract Manufacturers in Southern China
11. CAPACITY ANALYSIS
11.1. Chapter Overview
11.2. Assumptions and Methodology
11.3. Chinese Biopharmaceutical Contract Manufactures: Installed Capacity
11.3.1. Analysis by Company Size
11.3.2. Analysis by Scale of Operation
11.3.3. Analysis by Expression System Used
11.3.4. Analysis by Location of Manufacturing Facility
11.3.5. Analysis by Company Size and Location of Manufacturing Facility
11.3.6. Analysis by Scale of Operation and Location of Manufacturing Facility
11.4. Concluding Remarks
12. BIG PHARMA BIOPHARMACEUTICAL MANUFACTURING INITIATIVES IN CHINA
12.1. Chapter Overview
12.2. List of Biopharmaceutical R&D and Manufacturing Initiatives of Big Pharma Players in China
12.2.1. Analysis by Number of Initiatives
12.2.2. Analysis by Year of Initiative
12.2.3. Analysis by Company and Year of Initiative
12.2.4. Analysis by Type of Initiative
12.2.5. Analysis by Type of Biologic
12.3. Competitive Benchmarking of Big Pharmaceutical Players
12.3.1. Harvey Ball Analysis: Big Pharma Investment Summary
12.3.2. Geographical Analysis by Investment Made
13. MAKE VERSUS BUY DECISION MAKING FRAMEWORK
13.1. Chapter Overview
13.2. Assumptions and Key Parameters
13.3. Chinese Biopharmaceutical Contract Manufacturers: Make versus Buy Decision Making
13.3.1. Scenario 1
13.3.2. Scenario 2
13.3.3. Scenario 3
13.3.4. Scenario 4
13.4. Conclusion
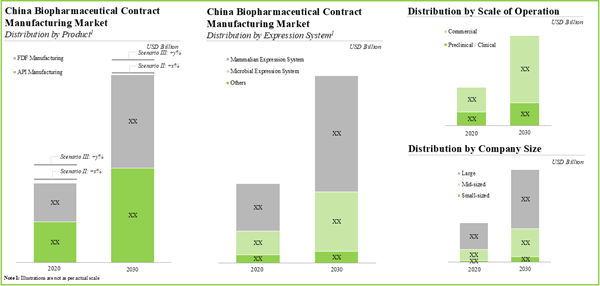
14. MARKET SIZING AND OPPORTUNITY ANALYSIS
14.1. Chapter Overview
14.2. Key Assumptions and Forecast Methodology
14.3. Biopharmaceutical Contract Manufacturing Market in China, Till 2035
14.3.1. Biopharmaceutical Contract Manufacturing Market in China for APIs, Till 2035
14.3.2. Biopharmaceutical Contract Manufacturing Market in China for FDFs, Till 2035
14.4. Biopharmaceutical Contract Manufacturing Market in China, Till 2035: Distribution by Expression System Used
14.4.1. Biopharmaceutical Contract Manufacturing Market in China, Till 2035: Share of Mammalian Systems
14.4.2. Biopharmaceutical Contract Manufacturing Market in China, Till 2035: Share of Microbial Systems
14.4.3. Biopharmaceutical Contract Manufacturing Market in China, Till 2035: Share of Other Expression Systems
14.5. Biopharmaceutical Contract Manufacturing Market in China, Till 2035: Distribution by Scale of Operation
14.5.1. Biopharmaceutical Contract Manufacturing Market in China, Till 2035: Share of Preclinical / Clinical Scale Operations
14.5.2. Biopharmaceutical Contract Manufacturing Market in China, Till 2035: Share of Commercial Operations
14.6. Biopharmaceutical Contract Manufacturing Market in China, Till 2035: Distribution by Company Size
14.6.1. Biopharmaceutical Contract Manufacturing Market in China, Till 2035: Share of Small Companies
14.6.2. Biopharmaceutical Contract Manufacturing Market in China, Till 2035: Share of Mid-sized Companies
14.6.3. Biopharmaceutical Contract Manufacturing Market in China, Till 2035: Share of Large and Very Large Companies
14.7. Biopharmaceutical Contract Manufacturing Market in China, Till 2035: Distribution by Type of Biologic
14.7.1. Biopharmaceutical Contract Manufacturing Market in China, Till 2035: Share of Antibodies
14.7.2. Biopharmaceutical Contract Manufacturing Market in China, Till 2035: Share of Vaccines
14.7.3. Biopharmaceutical Contract Manufacturing Market in China, Till 2035: Share of Other Biologics
15 COVID-19 Impact on China Biopharmaceutical CMO Market
15.1. Chapter Overview
15.2. Current Opinions and Recuperative Initiatives of Key Players
15.2.1. WuXi AppTec
15.2.2. Boehringer Ingelheim
15.2.3. GE Healthcare
15.2.4. Lonza
15.2.5. AmbioPharm
15.2.6. Impact on China Biopharmaceutical Contract Manufacturing Market
16. SWOT ANALYSIS
16.1. Chapter Overview
16.2. Strengths
16.3. Weaknesses
16.4. Opportunities
16.5. Threats
16.6. Comparison of SWOT Factors
16.7. Concluding Remarks
17. FUTURE OF THE CHINA BIOPHARMACEUTICAL CMO MARKET
17.1. Chapter Overview
17.2. Outsourcing Activities Anticipated to Increase in Future
17.3. Shift from One-time Contracts to Strategic Partnerships
17.4. Adoption of Innovative Technologies
17.4.1. Single Use Bioreactors
17.4.2. Novel Bioprocessing Techniques
17.4.3. Bioprocess Automation
17.5. Growing Popularity of the Quality by Design Principle in Bioprocessing
17.6. Increasing Focus on Niche Therapeutic Areas
17.7. Biosimilars Market to Contribute to Contract Service Revenues
17.8. Capability and Facility Expansions to Establish One Stop Shop Expertise
17.9. Increase in Financial In-flow and Outsourcing Budgets
17.10. Challenges Faced by Sponsors and Service Providers
17.10.1. Concerns Associated with Single Use Systems
17.10.2. Issues Related to Capacity Fluctuations
17.11. Concluding Remarks
LIST OF FIGURES
Figure 3.1. Types of Biopharmaceuticals
Figure 3.2. Key Steps Involved in the Manufacturing of Biopharmaceuticals
Figure 3.3. Type of Third-Party Service Providers
Figure 3.4. Chinese Regulatory Scenario: Responsibilities of the Contract Giver
Figure 3.5. Chinese Regulatory Scenario: Responsibilities of the Contract Acceptor
Figure 3.6. Important Aspects of the Contract
Figure 3.7. Commonly Outsourced Operations
Figure 3.8. Key Factors to Consider while Selecting a CMO Partner
Figure 3.9. Risks and Challenges Associated with Contract Manufacturing
Figure 4.1. Small Molecules versus Large Molecules: Comparison of Key Characteristics
Figure 4.2. Small Molecule versus Large Molecules: Comparison of Manufacturing Process
Figure 5.1. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Year of Establishment
Figure 5.2. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Company Size
Figure 5.3. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Scale of Operation
Figure 5.4. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Location of Headquarters
Figure 5.5. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Location of Manufacturing Facilities (Country-Wise)
Figure 5.6. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Type of Product
Figure 5.7. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Type of Services Offered
Figure 5.8. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Type of Biologic
Figure 5.9. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Type of Expression System Used
Figure 5.10. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Type of Bioreactor Used
Figure 5.11. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Mode of Operation of Bioreactor
Figure 5.12. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Type of Packaging Form Used
Figure 5.13. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Regulatory Accreditations / Certifications
Figure 6.1. JOINN Biologics: Development Services
Figure 6.2. WuXi Biologics: Annual Revenues, Since FY 2016 (RMB Million)
Figure 6.3. WuXi of Biologics: Revenues by Regions of Operation, Since FY 2019 (RMB Million)
Figure 7.1. Recent Partnerships: Cumulative Distribution by Year of Partnership
Figure 7.2. Recent Partnerships: Distribution by Partnership Model
Figure 7.3. Recent Partnerships: Year-wise Trend by Type of Partnership
Figure 7.4. Recent Partnerships: Distribution by Scale of Operation
Figure 7.5. Recent Partnerships: Distribution by Type of Biologic
Figure 7.6. Recent Partnerships: Distribution by Focus Area
Figure 7.7. Recent Partnerships: Distribution by Therapeutic Area
Figure 7.8. Recent Partnerships: Most Active Players
Figure 7.9. Geographical Distribution by Number of Partnerships
Figure 7.10. Geographical Distribution by Type of Partnership Model
Figure 7.11. Recent Partnerships: Intercontinental and Intracontinental Distribution
Figure 8.1. Recent Expansions: Cumulative Distribution by Year of Expansion, Since 2016
Figure 8.2. Recent Expansions: Distribution by Type of Expansion
Figure 8.3. Recent Expansions: Distribution by Scale of Operation
Figure 8.4. Recent Expansions: Distribution by Type of Biologic
Figure 8.5. Recent Expansions: Distribution by Type of Biologic and Type of Expansion
Figure 8.6. Recent Expansions: Distribution by Location of Expansion Project
Figure 8.7. Recent Expansions: Distribution by Type of Expansion and Location of Facility
Figure 8.8. Recent Expansions: Distribution by Capacity of Expanded Facility (L)
Figure 8.9. Recent Expansions: Most Active Players
Figure 8.10. Geographical Distribution by Number of Expansion
Figure 8.11. Geographical Distribution by Type of Expansion
Figure 8.12. Geographical Distribution by Country
Figure 9.1. Clinical Trials: Distribution by Trial Registration Year (Cumulative Year-wise Trend)
Figure 9.2. Clinical Trials: Distribution of Active Trials by Trial Phase
Figure 9.3. Clinical Trials: Distribution by Trial Recruitment Status
Figure 9.4. Clinical Trials: Distribution by Trial Registration Year and Trial Recruitment Status
Figure 9.5. Clinical Trial Analysis: Distribution by Geography
Figure 9.6. Clinical Trials: Distribution of Enrolled Patient Population by Geography
Figure 9.7. Clinical Trials: Distribution of Enrolled Patient Population by Trial Registration Year
Figure 9.8. Clinical Trials: Distribution of Enrolled Patient Population in Active Trials by Trial Phase
Figure 9.9. Clinical Trials: Distribution by Type of Sponsor / Collaborator
Figure 9.10. Clinical Trials: Most Active Industry Players (In Terms of Number of Registered Trials)
Figure 9.11. Clinical Trials: Most Active Non-Industry Players (In Terms of Number of Registered Trials)
Figure 9.12. Clinical Trials: Distribution by Clinical Trial Centers
Figure 10.1. Regional Distribution of Chinese Biopharmaceutical Contract Manufacturers by Location of Manufacturing Facilities
Figure 10.2. Regional Capability Analysis: Northern China
Figure 10.3. Regional Capability Analysis: Eastern China
Figure 10.4. Regional Capability Analysis: Eastern China
Figure 10.5. Regional Capability Analysis: Southern China
Figure 11.1. Chinese Biopharmaceutical Contract Manufacturing Capacity: Distribution by Range of Installed Capacity
Figure 11.2. Chinese Biopharmaceutical Contract Manufacturing Capacity: Distribution by Company Size
Figure 11.3. Chinese Biopharmaceutical Contract Manufacturing Capacity: Distribution by Scale of Operation
Figure 11.4. Chinese Biopharmaceutical Contract Manufacturing Capacity: Distribution by Type of Expression System Used
Figure 11.5. Chinese Biopharmaceutical Contract Manufacturing Capacity: Distribution by Location of Manufacturing Facility
Figure 11.6. Chinese Biopharmaceutical Contract Manufacturing Capacity: Distribution by Company Size and Location of Manufacturing Facility
Figure 11.7. Chinese Biopharmaceutical Contract Manufacturing Capacity: Distribution by Scale of Operation and Location of Manufacturing Facility
Figure 12.1. Big Pharma Players in China: Distribution by Number of Biopharmaceutical Manufacturing Focused Initiatives
Figure 12.2. Big Pharma Players in China: Cumulative Distribution by Year of Initiative
Figure 12.3. Big Pharma Players in China: Distribution by Company and Year of Initiative
Figure 12.4. Big Pharma Players in China: Distribution by Type of Initiative
Figure 12.5. Big Pharma Players in China: Distribution by Type of Biologic
Figure 12.6. Big Pharma Players in China: Distribution by Company and Type of Biologic
Figure 12.7. Harvey Ball Analysis: Big Pharma Investment Summary
Figure 12.8. Big Pharma Players in China: Distribution by Location of Initiative
Figure 12.9. Big Pharma Players in China: Regional Distribution of Initiatives
Figure 12.10. Big Pharma Players in China: Distribution by Company and Location of Initiative
Figure 13.1. Make versus Buy Decision Making Framework
Figure 13.2. Make versus Buy Decision Making: Possible Scenario Descriptions
Figure 14.1. Biopharmaceutical Contract Manufacturing Market in China, Till 2035 (USD Million)
Figure 14.2. Biopharmaceutical Contract Manufacturing Market in China for APIs, Till 2035 (USD Million)
Figure 14.3. Biopharmaceutical Contract Manufacturing Market in China for FDFs, Till 2035 (USD Million)
Figure 14.4. Biopharmaceutical Contract Manufacturing Market in China: Distribution by Type of Expression System Used (USD Billion)
Figure 14.5. Biopharmaceutical Contract Manufacturing Market in China: Share of Mammalian Systems, Till 2035 (USD Billion)
Figure 14.6. Biopharmaceutical Contract Manufacturing Market in China: Share of Microbial Systems, Till 2035 (USD Billion)
Figure 14.7. Biopharmaceutical Contract Manufacturing Market in China: Share of Other Expression Systems, Till 2035 (USD Billion)
Figure 14.8. Biopharmaceutical Contract Manufacturing Market in China: Distribution by Scale of Operation (USD Billion)
Figure 14.9. Biopharmaceutical Contract Manufacturing Market in China: Share of Preclinical / Clinical Scale Operations, Till 2035 (USD Billion)
Figure 14.10. Biopharmaceutical Contract Manufacturing Market in China: Share of Commercial Operations, Till 2035 (USD Billion)
Figure 14.11. Biopharmaceutical Contract Manufacturing Market in China: Distribution by Company Size, 2020, 2025 and 2030 (USD Billion)
Figure 14.12. Biopharmaceutical Contract Manufacturing Market in China: Share of Small Companies, Till 2035 (USD Billion)
Figure 14.13. Biopharmaceutical Contract Manufacturing Market in China: Share of Mid-sized Companies, Till 2035 (USD Billion)
Figure 14.14. Biopharmaceutical Contract Manufacturing Market in China: Share of Large / Very Large Companies, Till 2035 (USD Billion)
Figure 14.15. Biopharmaceutical Contract Manufacturing: Distribution of Manufacturing Agreements Based on Type of Biologics and Geography
Figure 14.16. Biopharmaceutical Contract Manufacturing Market in China: Distribution by Type of Biologic (USD Billion)
Figure 14.17. Biopharmaceutical Contract Manufacturing Market in China: Share of Antibodies, Till 2035 (USD Billion)
Figure 14.18. Biopharmaceutical Contract Manufacturing Market in China: Share of Vaccines, Till 2035 (USD Billion)
Figure 14.19. Biopharmaceutical Contract Manufacturing Market in China: Share of Other Biologics, Till 2035 (USD Billion)
Figure 15.1. Biopharmaceutical Contract Manufacturing Market in China, Till 2035: COVID-19 Impact Informed Scenario (USD Billion)
Figure 16.1. Chinese Biopharmaceutical Contract Manufacturers: SWOT Analysis
Figure 16.2. Comparison of SWOT Factors: Harvey Ball Analysis
LIST OF TABLES
Table 4.1. Small Molecules and Large Molecules: Comparison of Development Characteristics
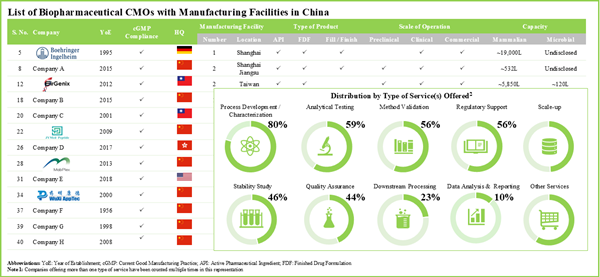
Table 5.1. List of Chinese Biopharmaceutical Contract Manufacturers
Table 5.2. Chinese Biopharmaceutical Contract Manufacturers: Information on Scale of Operation
Table 5.3. Chinese Biopharmaceutical Contract Manufacturers: Information on Type of Product
Table 5.4. Chinese Biopharmaceutical Contract Manufacturers: Information on Type of Services Offered
Table 5.5. Chinese Biopharmaceutical Contract Manufacturers: Information on Type of Biologic
Table 5.6. Chinese Biopharmaceutical Contract Manufacturers: Information on Type of Expression System Used
Table 5.7. Chinese Biopharmaceutical Contract Manufacturers: Information on Type of Bioreactor Used
Table 5.8. Chinese Biopharmaceutical Contract Manufacturers: Information on Mode of Operation of Bioreactor
Table 5.9. Chinese Biopharmaceutical Contract Manufacturers: Information on Type of Packaging Form Used
Table 5.10. Chinese Biopharmaceutical Contract Manufacturers: Information on Regulatory Accreditations
Table 6.1. ChemPartner Biologics: Company Overview
Table 6.2. ChemPartner Biologics: Overview of Manufacturing Capabilities
Table 6.3. ChemPartner Biologics: Recent Developments and Future Outlook
Table 6.4. JHL Biotech: Company Overview
Table 6.5. JHL Biotech: Overview of Manufacturing Capabilities
Table 6.6. JHL Biotech: Recent Developments and Future Outlook
Table 6.7. JOINN Biologics: Company Overview
Table 6.8. JOINN Biologics: Overview of Manufacturing Capabilities
Table 6.9. MabPlex: Company Overview
Table 6.10. MabPlex: Overview of Manufacturing Capabilities
Table 6.11. MabPlex: Recent Developments and Future Outlook
Table 6.12. Mycenax Biotech: Company Overview
Table 6.13. Mycenax Biotech: Overview of Manufacturing Capabilities
Table 6.14. Mycenax Biotech: Recent developments and Future Outlook
Table 6.15. WuXi AppTec: Company Overview
Table 6.16. WuXi Biologics: Overview of Manufacturing Capabilities
Table 6.17. WuXi Biologics: Recent Developments and Future Outlook
Table 7.1. Chinese Biopharmaceutical Contract Manufacturers: List of Partnerships, Since 2016
Table 8.1. Chinese Biopharmaceutical Contract Manufacturers: List of Expansions, Since 2016
Table 10.1. Chinese Biopharmaceutical Contract Manufacturers: Regional Distribution by Type of Product
Table 11.1 China Biopharmaceutical Contract Manufacturers: Installed Capacity
Table 11.2. Capacity Analysis: Average Capacity based on Company Size (Sample Dataset)
Table 11.3. Capacity Analysis: Total Installed Capacity in China
Table 18.1. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Year of Establishment
Table 18.2. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Company Size
Table 18.3. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Scale of Operation
Table 18.4. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Location of Headquarters
Table 18.5. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Location of Headquarters (Within China)
Table 18.6. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Location of Manufacturing Facilities (Country-Wise)
Table 18.7. Chinse Biopharmaceutical Contract Manufacturers: Distribution by Type of Product
Table 18.8. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Type of Services Offered
Table 18.9. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Type of Biologic
Table 18.10. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Type of Expression System Used
Table 18.11. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Type of Bioreactor Used
Table 18.12. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Mode of Operation of Bioreactor
Table 18.13. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Type of Packaging Form Used
Table 18.14. Chinese Biopharmaceutical Contract Manufacturers: Distribution by Regulatory Accreditations / Certifications
Table 18.15. WuXi Biologics: Annual Revenues, Since FY 2016 (RMB Million)
Table 18.16. WuXi Biologics: Revenues by Regions of Operation, Since FY 2019 (RMB Million)
Table 18.17. Recent Partnerships: Cumulative Distribution by Year of Partnership
Table 18.18. Recent Partnerships: Distribution by Partnership Model
Table 18.19. Recent Partnerships: Year-wise Trend by Type of Partnership
Table 18.20. Recent Partnerships: Distribution by Scale of Operation
Table 18.21. Recent Partnerships: Distribution by Type of Biologic
Table 18.22. Recent Partnerships: Distribution by Focus Area
Table 18.23. Recent Partnerships: Distribution by Therapeutic Area
Table 18.24. Recent Partnerships: Most Active Players
Table 18.25. Geographical Distribution by Number of Partnerships
Table 18.26. Geographical Distribution by Type of Partnership Model
Table 18.27. Recent Partnerships: Intercontinental and Intracontinental Distribution
Table 18.28. Recent Expansions: Cumulative Distribution by Year of Expansion, Since 2016
Table 18.29. Recent Expansions: Distribution by Type of Expansion
Table 18.30. Recent Expansions: Distribution by Sale of Operation
Table 18.31. Recent Expansions: Distribution by Type of Biologic
Table 18.32. Recent Expansions: Distribution by Type of Biologic and Type of Expansion
Table 18.33. Recent Expansions: Distribution by Location of Expansion Project
Table 18.34. Recent Expansions: Distribution by Type of Expansion and Location of Facility
Table 18.35. Recent Expansions: Distribution by Capacity of Expanded Facility (L)
Table 18.36. Recent Expansions: Most Active Players
Table 18.37. Geographical Distribution by Number of Expansions
Table 18.38. Geographical Distribution by Type of Expansion
Table 18.39. Recent Expansions: Geographical Distribution by Country
Table 18.40. Clinical Trials: Distribution by Trial Registration Year (Cumulative Year-wise Trend)
Table 18.41. Clinical Trials: Distribution of Active Trials by Trial Phase
Table 18.42. Clinical Trials: Distribution by Trial Recruitment Status
Table 18.43. Clinical Trials: Distribution by Trial Registration Year and Trial Recruitment Status
Table 18.44. Clinical Trials: Distribution by Geography
Table 18.45. Clinical Trials: Distribution of Enrolled Patient Population by Geography
Table 18.46. Clinical Trials: Distribution of Enrolled Patient Population by Trial
Table 18.47. Clinical Trials: Distribution of Enrolled Patient Population in Active Trials by Trial Phase
Table 18.48. Clinical Trials: Distribution by Type of Sponsor / Collaborator
Table 18.49. Clinical Trials: Most Active Industry Players (In terms of Number of Registered Trials)
Table 18.50. Clinical Trials: Most Active Non-Industry Players (In terms of Number of Registered Trials)
Table 18.51. Clinical Trials: Distribution by Clinical Trial Centers
Table 18.52. Regional Distribution of Chinese Biopharmaceutical Contract Manufacturers by Location of Manufacturing Facilities
Table 18.53. Chinese Biopharmaceutical Contract Manufacturers: Regional Distribution by Type of Product
Table 18.54. Regional Capability Analysis: Northern China
Table 18.55. Regional Capability Analysis: Eastern China
Table 18.56. Regional Capability Analysis: Central China
Table 18.57. Regional Capability Analysis: Southern China
Table 18.58. Chinese Biopharmaceutical Contract Manufacturing Capacity: Distribution by Range of Installed Capacity
Table 18.59. Chinese Biopharmaceutical Contract Manufacturing Capacity: Distribution by Company Size
Table 18.60. Chinese Biopharmaceutical Contract Manufacturing Capacity: Distribution by Scale of Operation
Table 18.61. Chinese Biopharmaceutical Contract Manufacturing Capacity: Distribution by Type of Expression System Used
Table 18.62. Chinese Biopharmaceutical Contract Manufacturing Capacity: Distribution by Location of Manufacturing Facility
Table 18.63. Chinese Biopharmaceutical Contract Manufacturing Capacity: Distribution by Company Size and Location of Manufacturing Facility
Table 18.64. Chinese Biopharmaceutical Contract Manufacturing Capacity: Distribution by Scale of Operation and Location of Manufacturing Facility
Table 18.65. Big Pharma Players in China: Distribution by Number of Biopharmaceutical Manufacturing Focused Initiatives
Table 18.66. Big Pharma Players in China: Cumulative Distribution by Year of Initiative
Table 18.67. Big Pharma Players in China: Distribution by Company and Year of Initiative
Table 18.68. Big Pharma Players in China: Distribution by Type of Initiative
Table 18.69. Big Pharma Players in China: Distribution by Type of Biologic
Table 18.70. Big Pharma Players in China: Distribution by Company and Type of Biologic
Table 18.71. Harvey Ball Analysis: Big Pharma Investment Summary
Table 18.72. Big Pharma Players in China: Distribution by Location of Initiative
Table 18.73. Big Pharma Players in China: Regional Distribution of Initiatives
Table 18.74. Big Pharma Players in China: Distribution by Company and Location of Initiative
Table 18.75. Biopharmaceutical Contract Manufacturing Market in China, Till 2035 (USD Billion)
Table 18.76. Biopharmaceutical Contract Manufacturing Market in China for APIs, Till 2035 (USD Billion)
Table 18.77. Biopharmaceutical Contract Manufacturing Market in China for FDFs, Till 2035 (USD Billion)
Table 18.78. Biopharmaceutical Contract Manufacturing Market in China: Distribution by Type of Expression System Used
Table 18.79. Biopharmaceutical Contract Manufacturing Market in China: Share of Mammalian Systems, Till 2035 (USD Billion)
Table 18.80. Biopharmaceutical Contract Manufacturing Market in China: Share of Microbial Systems, Till 2035 (USD Billion)
Table 18.81. Biopharmaceutical Contract Manufacturing Market in China: Share of Other Expression Systems, Till 2035 (USD Billion)
Table 18.82. Biopharmaceutical Contract Manufacturing Market in China: Distribution by Scale of Operation (USD Billion)
Table 18.83. Biopharmaceutical Contract Manufacturing Market in China: Share of Preclinical / Clinical Scale Operations, Till 2035 (USD Billion)
Table 18.84. Biopharmaceutical Contract Manufacturing Market in China: Share of Commercial Scale of Operations, Till 2035 (USD Billion)
Table 18.85. Biopharmaceutical Contract Manufacturing Market in China: Distribution by Company Size (USD Billion)
Table 18.86. Biopharmaceutical Contract Manufacturing Market in China: Share of Small Companies, Till 2035 (USD Billion)
Table 18.87. Biopharmaceutical Contract Manufacturing Market in China: Share of Mid-sized Companies, Till 2035 (USD Billion)
Table 18.88. Biopharmaceutical Contract Manufacturing Market in China: Share of Large and Very Large Companies, Till 2035 (USD Billion)
Table 18.89. Biopharmaceutical Contract Manufacturing Market in China: Distribution by Type of Biologic (USD Billion)
Table 18.90. Biopharmaceutical Contract Manufacturing Market in China: Share of Antibodies, Till 2035 (USD Billion)
Table 18.91. Biopharmaceutical Contract Manufacturing Market in China: Share of Vaccines, Till 2035 (USD Billion)
Table 18.92. Biopharmaceutical Contract Manufacturing Market in China: Share of Others, Till 2035 (USD Billion)
Table 18.93. Biopharmaceutical Contract Manufacturing Market in China, Till 2035: COVID-19 Impact Informed Scenario (USD Billion)