Executive Summary and Market Analysis
The South and Central America medical writing market is primarily divided into three regions: Brazil, Argentina, and the Rest of South and Central America. The expansion of this market is largely fueled by the burgeoning pharmaceutical and biotechnology industries, an increase in clinical trials, and the need for compliance with stringent regulatory standards. As companies strive for regulatory adherence and high-quality documentation necessary for drug approvals, the demand for specialized medical writing services is on the rise throughout the region.Market Segmentation Analysis
The medical writing market can be segmented based on type, application, and end user.- By Type: The market is categorized into clinical writing, regulatory writing, and scientific writing. In 2023, clinical writing accounted for the largest share of the market, reflecting its critical role in the documentation of clinical trials and studies.
- By Application: The applications of medical writing include medical journalism, medical education, and medico marketing. Medical journalism held the largest market share in 2023, indicating a strong demand for content that communicates medical information effectively to the public and healthcare professionals.
- By End User: The market is divided into pharmaceutical and biotechnology companies, and contract research organizations (CROs). In 2023, pharmaceutical and biotechnology companies represented a larger share of the market, highlighting their reliance on medical writing for regulatory submissions and clinical documentation.
Market Outlook
The increasing need for regulatory and clinical trial documentation, driven by advancements in personalized medicine and biologics, is expected to create significant opportunities within the medical writing sector. As the healthcare landscape shifts towards personalized treatments, there is a growing requirement for precise and tailored documentation that meets regulatory standards and facilitates scientific communication. The rise of biologics, gene therapies, and cell-based treatments presents a notable opportunity for medical writers with expertise in these specialized areas.Moreover, the expansion of pharmaceutical companies and evolving healthcare policies are contributing to the growth of medical writing services in emerging economies, particularly in Brazil. With more pharmaceutical companies conducting clinical trials in Latin America, there is an increasing demand for writers who are knowledgeable about local regulatory requirements and can produce documentation in multiple languages. Additionally, the digital transformation of the healthcare sector is opening new avenues for medical writers to create content for eHealth and digital health platforms, including telemedicine and mobile health applications. As digital health technologies gain traction, there is a pressing need for skilled writers to develop clear and informative content that explains medical treatments, guidelines, and data to both healthcare professionals and patients.
Country Insights
The South and Central America medical writing market is primarily composed of Brazil, Argentina, and the Rest of South and Central America, with Brazil holding the largest market share in 2023.Brazil stands out as the largest pharmaceutical market in the region, with key players such as EMS and Aché Laboratórios expanding their operations. This growth leads to an increase in regulatory submissions, clinical trial documentation, and product labeling, thereby heightening the demand for medical writing services. In some cases, these companies also engage medical writers from outside Brazil to ensure high-quality documentation. The National Health Surveillance Agency (ANVISA) is the regulatory body overseeing clinical trials, inspections, and drug approvals in Brazil. ANVISA enforces strict guidelines, which amplifies the need for specialized medical writers who can ensure compliance with both local and international standards. The rising number of clinical trials, especially in therapeutic areas like oncology, cardiology, and infectious diseases, further drives the demand for clinical study reports, trial protocols, and informed consent forms. Major market players such as Parexel, Covance, and IQVIA have established a strong presence in Brazil, providing medical writing services tailored to meet local requirements.
Company Profiles
Key players in the South and Central America medical writing market include Cactus Communications, Parexel International Corp, Certara Inc., Freyr LLC, Quanticate International Ltd, SIRO Clinpharm Pvt Ltd, ProPharma Group, Caidya, Wipro Ltd, SGS SA, Laboratory Corp of America Holdings, TUNECT, Indegene Ltd, Evidera, Syner-G BioPharma Group, EMTEX BV, BioAgile Therapeutics Private Limited, Tata Consultancy Services Ltd, InClin, and TransPerfect Life Sciences, among others. These companies are employing various strategies, including expansion, product innovation, and mergers and acquisitions, to enhance their market share and deliver innovative solutions to their clients.Table of Contents
Companies Mentioned
- Cactus Communications
- Parexel International Corp
- Certara Inc
- Freyr LLC
- Quanticate International Ltd
- SIRO Clinpharm Pvt Ltd
- ProPharma Group
- Caidya
- Wipro Ltd
- SGS SA
- Laboratory Corp of America Holdings
- TUNECT
- Indegene Ltd
- Evidera
- Syner-G BioPharma Group
- EMTEX BV
- BioAgile Therapeutics Private Limited
- Tata Consultancy Services Ltd
- InClin
- TransPerfect Life Sciences
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 173 |
| Published | July 2025 |
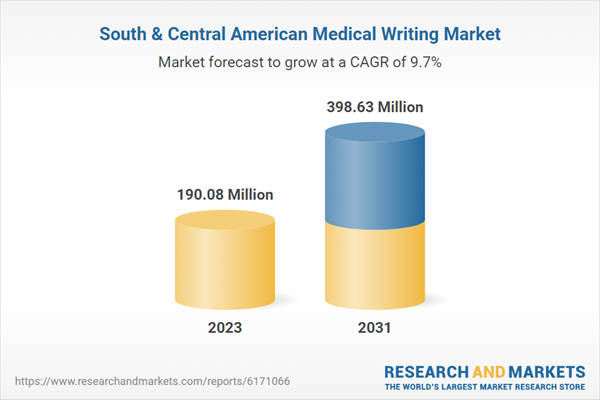
| Forecast Period | 2023 - 2031 |
| Estimated Market Value in 2023 | 190.08 Million |
| Forecasted Market Value by 2031 | 398.63 Million |
| Compound Annual Growth Rate | 9.7% |
| No. of Companies Mentioned | 20 |