Executive Summary and Market Analysis
The gastrointestinal drugs market in North America is divided into three primary regions: the United States, Canada, and Mexico. Several factors are driving the growth of this market, including the rising incidence of gastrointestinal disorders linked to lifestyle changes, government initiatives aimed at the prevention and treatment of these diseases, a robust healthcare infrastructure, a thriving pharmaceutical sector, comprehensive reimbursement coverage, and the presence of major pharmaceutical companies.Market Segmentation Analysis
The North America gastrointestinal drugs market can be analyzed through various segments, including drug class, application, route of administration, and distribution channel.1. Drug Class: The market is categorized into several drug classes, including biologics, antidiarrheal and laxatives, acid neutralizers, anti-inflammatory drugs, antiemetic and antinauseants, among others. In 2023, biologics accounted for the largest market share, reflecting their growing importance in treating gastrointestinal conditions.
2. Application: The market is also segmented by application, which includes conditions such as irritable bowel syndrome (IBS), inflammatory bowel diseases (IBD) like ulcerative colitis and Crohn's disease, gastroenteritis, celiac disease, and others. The IBS segment held the largest share in 2023, indicating a significant demand for treatments targeting this condition.
3. Route of Administration: The gastrointestinal drugs market is divided into oral and parenteral routes of administration. The oral segment dominated the market in 2023, as oral medications are often preferred for their convenience and ease of use.
4. Distribution Channel: The distribution of gastrointestinal drugs is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. Hospital pharmacies represented the largest share of the market in 2023, highlighting the critical role of hospitals in dispensing these medications.
Market Outlook
Recent years have seen a surge in the development of advanced treatments, particularly biologics, which are increasingly used for managing inflammatory diseases. Biologics have revolutionized the treatment landscape for inflammatory bowel diseases (IBD), and their success has encouraged their application in other gastrointestinal disorders. These drugs target specific elements of the immune system that contribute to disease, making them effective for conditions such as eosinophilic esophagitis, celiac disease, and autoimmune hepatitis.Biologics, particularly anti-TNF agents, have been pivotal in managing ulcerative colitis and Crohn's disease. These agents, which include well-known medications like Humira, Simponi, and Remicade, work by blocking tumor necrosis factor-alpha (TNF-alpha), a protein that promotes inflammation. The introduction of these biologics has significantly improved the management of IBD, with many patients achieving remission through their use. The FDA has approved numerous biologic drugs for IBD treatment, including Humira, Cimzia, Simponi, Tysabri, Remicade, Entyvio, and Stelara. Notably, Vedolizumab has emerged as a preferred second-line biologic for moderate to severe cases of Crohn's disease and ulcerative colitis.
In February 2024, Celltrion Healthcare’s Remsima SC received approval from Health Canada for maintenance therapy in IBD, further illustrating the ongoing innovation in this field. The increasing prevalence of gastrointestinal diseases in developing countries is also driving the demand for new biologics, contributing to market growth.
Country Insights
Geographically, the North American gastrointestinal drugs market is primarily composed of the US, Canada, and Mexico, with the US holding the largest market share in 2023. The rising prevalence of gastrointestinal diseases in the US is a significant factor driving demand for these drugs. Conditions such as Crohn's disease and ulcerative colitis are particularly prevalent, with approximately 70,000 new cases diagnosed annually, according to the Crohn’s & Colitis Foundation of America. The overall prevalence of IBD has increased notably from 2011 to 2020, with estimates suggesting that around 2.4 million Americans are affected.Increased funding for research and development in gastrointestinal diseases is expected to enhance the focus on novel treatment options. For instance, Crohn's disease research received US$ 90 million in funding in 2022, underscoring the commitment to advancing treatment options.
The US FDA's approval of new drugs is also anticipated to bolster market growth. For example, in May 2020, the FDA approved Qinlock for advanced gastrointestinal stromal tumors, and in May 2024, Strides Pharma received approval for a generic version of Sucralfate Oral Suspension for various gastrointestinal conditions.
Company Profiles
Key players in the North America gastrointestinal drugs market include Sanofi SA, GSK Plc, Johnson & Johnson, Bausch Health Companies Inc, AstraZeneca Plc, Takeda Pharmaceutical Co Ltd, AbbVie Inc, Bayer AG, Celltrion Inc, and Pfizer Inc. These companies are employing various strategies, including expansion, product innovation, and mergers and acquisitions, to enhance their market presence and offer innovative solutions to consumers.Table of Contents
Companies Mentioned
- Sanofi SA
- GSK Plc
- Johnson & Johnson
- Bausch Health Companies Inc
- AstraZeneca Plc
- Takeda Pharmaceutical Co Ltd
- AbbVie Inc
- Bayer AG
- Celltrion Inc
- Pfizer Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 155 |
| Published | July 2025 |
| Forecast Period | 2023 - 2031 |
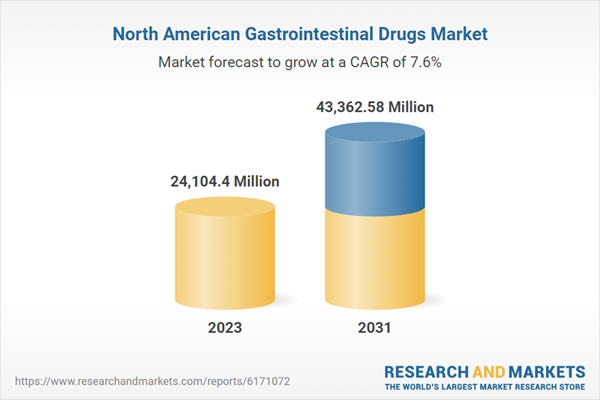
| Estimated Market Value in 2023 | 24104.4 Million |
| Forecasted Market Value by 2031 | 43362.58 Million |
| Compound Annual Growth Rate | 7.6% |
| Regions Covered | North America |
| No. of Companies Mentioned | 10 |