Executive Summary and Market Analysis
The medical writing sector in North America is primarily driven by several factors, including increasing regulatory requirements, a rise in the number of clinical trials, and the expansion of the pharmaceutical and biotechnology industries. The demand for precise medical documentation is further fueled by stringent compliance standards set by regulatory bodies such as the United States Food and Drug Administration (US FDA). Additionally, the trend of outsourcing medical writing tasks is contributing to the market's growth.Market Segmentation Analysis
The North American medical writing market can be segmented based on type, application, and end user.1. By Type: The market is divided into clinical writing, regulatory writing, and scientific writing. In 2023, clinical writing held the largest market share, reflecting its critical role in the documentation of clinical trials and regulatory submissions.
2. By Application: The applications of medical writing include medical journalism, medical education, and medico marketing. Medical journalism was the leading segment in 2023, highlighting the importance of accurate and engaging communication in the medical field.
3. By End User: The market is categorized into pharmaceutical and biotechnology companies and contract research organizations (CROs). In 2023, pharmaceutical and biotechnology companies accounted for a larger share of the market, driven by their need for comprehensive documentation throughout the drug development process.
Market Outlook
Regulatory authorities require detailed documentation of methodologies used during all phases of product development to approve new products in the biopharmaceutical and pharmaceutical sectors. Insurance providers also demand thorough information about drugs to establish reimbursement policies. This creates a significant challenge for pharmaceutical and biopharmaceutical companies to adhere to industry standards.Medical writers play a vital role in this process, as they are involved in various tasks that provide insights into protocol design. The data generated from clinical trials must be accurately reflected in product labeling and summarized in a way that is understandable, avoiding technical jargon. Medical writers are adept at processing and analyzing information according to regulatory requirements, which helps expedite the document development process and, consequently, the overall drug development timeline. This efficiency is crucial for obtaining regulatory approvals smoothly, thereby increasing the importance of medical writing in the industry.
Country Insights
The North American medical writing market includes the United States, Canada, and Mexico, with the US holding the largest market share in 2023.The growth of the US medical writing market is driven by several key factors:
- Regulatory Demands: The surge in drug approvals, including 55 new drugs approved by the Center for Drug Evaluation and Research (CDER) in 2023, has increased the need for accurate regulatory documentation. Medical writers are essential in preparing clinical study reports, regulatory submissions, and other compliance documents.
- Clinical Trials Growth: The US is a leading hub for clinical trials, particularly in areas such as oncology, rare diseases, and immunology. The increasing number of clinical trials necessitates the support of medical writers for trial protocols, regulatory filings, and clinical reports.
- Pharmaceutical and Biotech Expansion: The presence of major pharmaceutical companies like Pfizer, Johnson & Johnson, and Merck in the US drives the demand for regulatory submissions, clinical trial reports, and product labeling, further expanding the medical writing market.
Key Players in the Market
Prominent players in the North American medical writing market include IQVIA, Parexel, Syneos Health, and Covance. These companies are employing various strategies for market expansion, including partnerships and collaborations. For instance, Parexel has formed a strategic alliance with Palantir Technologies Inc., a leader in artificial intelligence systems, to enhance the efficiency and safety of clinical trials for biopharmaceutical clients. This collaboration aims to leverage Palantir’s technology to improve Parexel’s clinical data platform, thereby streamlining the clinical trial process while maintaining high standards of safety and regulatory compliance.Company Profiles
Key companies operating in the North American medical writing market include Cactus Communications, Parexel International Corp, Certara Inc., Freyr LLC, Quanticate International Ltd, SIRO Clinpharm Pvt Ltd, ProPharma Group, Caidya, Wipro Ltd, SGS SA, Laboratory Corp of America Holdings, TUNECT, Indegene Ltd, Evidera, Syner-G BioPharma Group, EMTEX BV, BioAgile Therapeutics Private Limited, Tata Consultancy Services Ltd, InClin, and TransPerfect Life Sciences. These companies are pursuing various strategies such as expansion, product innovation, and mergers and acquisitions to enhance their offerings and increase their market share.Table of Contents
Companies Mentioned
- Cactus Communications
- Parexel International Corp
- Certara Inc
- Freyr LLC
- Quanticate International Ltd
- SIRO Clinpharm Pvt Ltd
- ProPharma Group
- Caidya
- Wipro Ltd
- SGS SA
- Laboratory Corp of America Holdings
- TUNECT
- Indegene Ltd
- Evidera
- Syner-G BioPharma Group
- EMTEX BV
- BioAgile Therapeutics Private Limited
- Tata Consultancy Services Ltd
- InClin
- TransPerfect Life Sciences
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 175 |
| Published | July 2025 |
| Forecast Period | 2023 - 2031 |
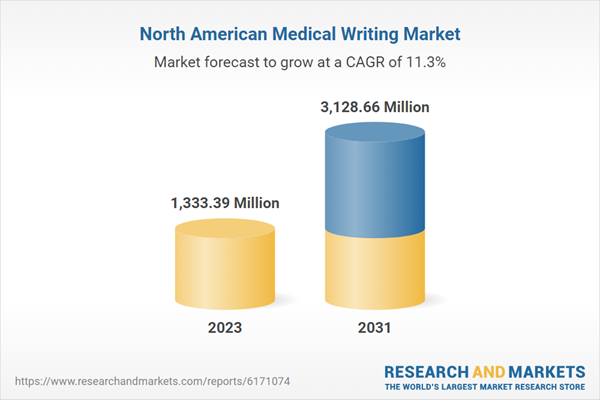
| Estimated Market Value in 2023 | 1333.39 Million |
| Forecasted Market Value by 2031 | 3128.66 Million |
| Compound Annual Growth Rate | 11.3% |
| Regions Covered | North America |
| No. of Companies Mentioned | 20 |