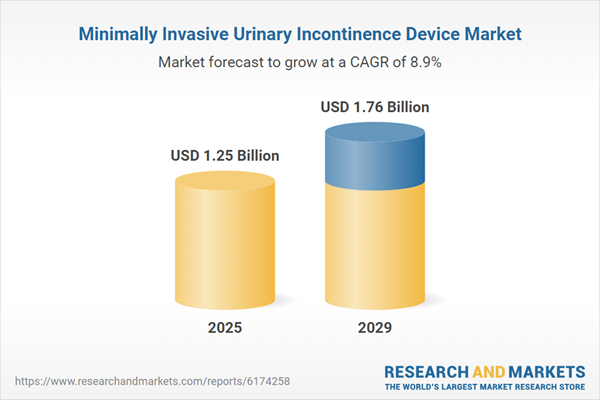
The minimally invasive urinary incontinence device market size is expected to see strong growth in the next few years. It will grow to $1.76 billion in 2029 at a compound annual growth rate (CAGR) of 8.9%. The growth in the forecast period can be attributed to increasing reimbursement support for minimally invasive urinary treatments, growing demand for outpatient and same-day procedures, a rising focus on patient comfort and faster recovery, expanding adoption in emerging markets, and a higher incidence of incontinence due to lifestyle and medical factors. Major trends in the forecast period include ongoing innovations in device miniaturization and material safety, integration of wireless monitoring and control features, advancements in nerve stimulation technologies, development of adjustable and reusable sling systems, and the use of 3D printing for customized urinary devices.
The growing incidence of prostate cancer is expected to drive the growth of the minimally invasive urinary incontinence device market in the coming years. Prostate cancer, characterized by uncontrolled cell growth in the prostate gland, can lead to significant urinary complications and reduced quality of life. The rising prevalence of the disease is largely attributed to an aging male population, as older age is a major risk factor. Minimally invasive urinary incontinence devices help manage prostate cancer-related urinary issues by providing targeted support to control bladder leakage, restore normal urinary function, and improve patient quality of life. For example, in June 2025, the U.S. Centers for Disease Control and Prevention reported an estimated 255,395 new cases of prostate cancer in 2022, with 33,881 deaths in 2023, highlighting the increasing patient population driving demand for effective urinary incontinence solutions.
Companies in the minimally invasive urinary incontinence device market are focusing on innovations such as neuromodulation therapy to provide effective, long-term symptom management with minimal surgical intervention. Neuromodulation therapy devices are implants that stimulate bladder-controlling nerves to regulate urinary function, helping reduce leakage and improve bladder control. For instance, in December 2023, BlueWind Medical Ltd., a U.S.-based neurostimulation company, launched its Revi implantable tibial neuromodulation (iTNM) device. This battery-free, minimally invasive implant is placed near the ankle during a single outpatient procedure under local anesthesia and is activated via a wearable device, enabling patients to conveniently manage urge urinary incontinence (UUI) at home. This launch exemplifies the trend toward patient-friendly, technologically advanced neuromodulation solutions.
In November 2024, Boston Scientific Corporation acquired Axonics Inc. for approximately \$3.7 billion to expand its minimally invasive urinary incontinence portfolio. This acquisition allows Boston Scientific to strengthen its presence in the high-growth sacral neuromodulation segment, integrate Axonics’ innovative technologies, and offer a comprehensive range of treatment options for urinary and bowel dysfunction. Axonics Inc., a U.S.-based medical technology company, specializes in minimally invasive urinary incontinence devices, and this strategic move is expected to drive long-term growth in the urology device market.
Major players in the minimally invasive urinary incontinence device market are Johnson & Johnson Services Inc., Medtronic plc, Becton Dickinson and Company, Stryker Corporation, Boston Scientific Corporation, Olympus Corporation, Coloplast A/S, Teleflex Incorporated, Hollister Incorporated, CooperSurgical Inc., B. Braun Melsungen Aktiengesellschaft, LiNA Medical ApS, BlueWind Medical Ltd., Caldera Medical Inc., Neuspera Medical Inc., InControl Medical LLC, UroMems Inc., Rigicon Inc., Valencia Technologies Corporation, Zephyr Surgical Implants SA.
North America was the largest region in the minimally invasive urinary incontinence device market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in minimally invasive urinary incontinence device market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the minimally invasive urinary incontinence device market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The fast surge in U.S. tariffs and the trade tensions that followed in spring 2025 are heavily affecting the medical equipment sector, particularly for imported imaging machine components, surgical-grade stainless steel, and plastic disposables. Hospitals and clinics resist price hikes, pressuring manufacturers’ margins. Regulatory hurdles compound the problem, as tariff-related supplier changes often require re-certification of devices, delaying time-to-market. Companies are mitigating risks by dual-sourcing critical parts, expanding domestic production of commoditized items, and accelerating R&D in cost-efficient materials.
A minimally invasive urinary incontinence device is a medical tool designed to treat or manage urinary incontinence using techniques that minimize surgical trauma, shorten recovery time, and reduce complication risks compared to traditional open procedures. These devices function by supporting the urethra, improving sphincter function, or modulating nerve activity to enhance bladder control, and are typically implanted or applied through small incisions, injections, or percutaneous methods.
The primary types of minimally invasive urinary incontinence devices include tension-free vaginal tape (TVT), tension-free vaginal tape obturator system (TVTO), and other variants. Tension-free vaginal tape (TVT) is a minimally invasive procedure that uses a synthetic mesh to support the urethra and prevent stress urinary incontinence in women. These devices are used by adults (18-65 years), seniors (65 years and above), and pediatric patients, and are employed across multiple end-users, including hospitals, clinics, home healthcare settings, and research institutions.
The minimally invasive urinary incontinence device market research report is one of a series of new reports that provides minimally invasive urinary incontinence device market statistics, including minimally invasive urinary incontinence device industry global market size, regional shares, competitors with the minimally invasive urinary incontinence device market share, minimally invasive urinary incontinence device market segments, market trends, and opportunities, and any further data you may need to thrive in the minimally invasive urinary incontinence device industry. This minimally invasive urinary incontinence device market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The minimally invasive urinary incontinence device market consists of sales of wearable devices, catheter-based devices, sling systems, adjustable balloon systems and bulking agent delivery systems. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Minimally Invasive Urinary Incontinence Device Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on minimally invasive urinary incontinence device market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for minimally invasive urinary incontinence device? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The minimally invasive urinary incontinence device market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Tension Free Vaginal Tape (TVT); Tension Free Vaginal Tape (TVT) Obturator System; Other Types2) By Age Group: Adults (18-65 years); Senior Population (65 Years and Above); Pediatric Population
3) By End-User: Hospitals; Clinics; Home Healthcare Settings; Research Institutions
Subsegments:
1) By Tension Free Vaginal Tape (TVT): Mid Urethral Sling; Retropubic Sling; Single Incision Sling2) By Tension Free Vaginal Tape Obturator System (TVTO): Inside Out Approach; Outside in Approach
3) By Other Types: Bulking Agents; Artificial Urinary Sphincter; Sling Systems
Companies Mentioned: Johnson & Johnson Services Inc.; Medtronic plc; Becton Dickinson and Company; Stryker Corporation; Boston Scientific Corporation; Olympus Corporation; Coloplast A/S; Teleflex Incorporated; Hollister Incorporated; CooperSurgical Inc.; B. Braun Melsungen Aktiengesellschaft; LiNA Medical ApS; BlueWind Medical Ltd.; Caldera Medical Inc.; Neuspera Medical Inc.; InControl Medical LLC; UroMems Inc.; Rigicon Inc.; Valencia Technologies Corporation; Zephyr Surgical Implants SA
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Minimally Invasive Urinary Incontinence Device market report include:- Johnson & Johnson Services Inc.
- Medtronic plc
- Becton Dickinson and Company
- Stryker Corporation
- Boston Scientific Corporation
- Olympus Corporation
- Coloplast A/S
- Teleflex Incorporated
- Hollister Incorporated
- CooperSurgical Inc.
- B. Braun Melsungen Aktiengesellschaft
- LiNA Medical ApS
- BlueWind Medical Ltd.
- Caldera Medical Inc.
- Neuspera Medical Inc.
- InControl Medical LLC
- UroMems Inc.
- Rigicon Inc.
- Valencia Technologies Corporation
- Zephyr Surgical Implants SA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.25 Billion |
| Forecasted Market Value ( USD | $ 1.76 Billion |
| Compound Annual Growth Rate | 8.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |