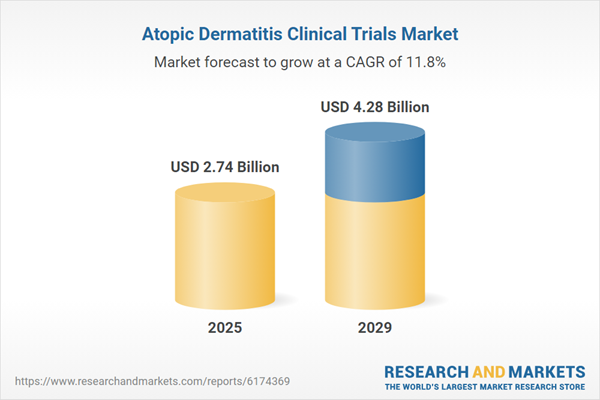
The atopic dermatitis clinical trials market size is expected to see rapid growth in the next few years. It will grow to $4.28 billion in 2029 at a compound annual growth rate (CAGR) of 11.8%. The growth in the forecast period can be attributed to the rising adoption of biologic therapies, increasing investments in research and development, growing patient enrollment in clinical trials, expansion of healthcare infrastructure, and stronger regulatory support for dermatology studies. Major trends in the forecast period include advancements in personalized medicine, innovations in biologic treatments, integration of digital health technologies, implementation of advanced clinical trial designs, and the increasing use of real-world evidence.
The increasing pharmaceutical and biopharma investments are expected to propel the growth of the atopic dermatitis clinical trials market going forward. Pharmaceutical and biopharma investments refer to capital allocated toward the research, development, manufacturing, and commercialization of drugs and biologic therapies. These investments are rising due to growing demand for innovative therapies that provide targeted treatment options and improved patient outcomes. Pharmaceutical and biopharma investments support atopic dermatitis clinical trials by funding research and testing of new treatments, enabling comprehensive studies to confirm safety and efficacy. For instance, in March 2022, according to the Australian Government Department of Health, Disability and Ageing, the government committed \$45.5 billion over four years for affordable medicines through the Pharmaceutical Benefits Scheme, as part of a broader \$537 billion four-year health investment, including a \$2.4 billion boost for adding new medicines. Therefore, the increasing pharmaceutical and biopharma investments are driving the growth of the atopic dermatitis clinical trials market.
Major companies in the atopic dermatitis clinical trials market are focusing on innovative products, such as oral inhibitors, to maintain stable therapeutic effects over time. Oral inhibitors are drugs administered by mouth that block enzymes or signaling pathways involved in disease progression. For example, in February 2023, Pfizer Inc., a US-based pharmaceutical and biotechnology company, announced that the U.S. Food and Drug Administration approved its supplemental New Drug Application for CIBINQO (abrocitinib) for adolescents aged 12 to under 18 with moderate-to-severe atopic dermatitis not controlled by other systemic treatments or biologics. Previously, CIBINQO was approved only for adults 18 and older, providing a new treatment option for younger patients with difficult-to-treat AD.
In March 2022, Pfizer Inc. acquired Arena Pharmaceuticals for $6.7 billion to strengthen its inflammation and immunology portfolio, primarily through Arena’s key asset, etrasimod. Etrasimod is under development for multiple immune-mediated conditions, including atopic dermatitis, ulcerative colitis, and Crohn’s disease. Arena Pharmaceuticals is a US-based biopharmaceutical company actively conducting clinical trials in atopic dermatitis.
Major players in the atopic dermatitis clinical trials market are Pfizer Inc., Johnson & Johnson, AbbVie Inc., Novartis International AG, Sanofi S.A., GlaxoSmithKline Plc, Eli Lilly and Company, Amgen Inc., Regeneron Pharmaceuticals Inc., Organon & Co., Charles River Laboratories International Inc., LEO Pharma A/S, QIMA Limited, Medpace Holdings Inc., Novotech Health Holdings Pte Ltd., Biocytogen Pharmaceuticals Co. Ltd., REPROCELL Inc., AnaptysBio Inc., Imavita S.A.S., and Oncodesign Services S.A.S.
North America was the largest region in the atopic dermatitis clinical trials market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in atopic dermatitis clinical trials market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the atopic dermatitis clinical trials market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the consequent trade frictions in spring 2025 are severely impacting the healthcare sector, particularly in the supply of critical medical devices, diagnostic equipment, and pharmaceuticals. Hospitals and healthcare providers are facing higher costs for imported surgical instruments, imaging equipment, and consumables such as syringes and catheters, many of which have limited domestic alternatives. These increased costs are straining healthcare budgets, leading some providers to delay equipment upgrades or pass on expenses to patients. Additionally, tariffs on raw materials and components are disrupting the production of essential drugs and devices, causing supply chain bottlenecks. In response, the industry is diversifying sourcing strategies, boosting local manufacturing where possible, and advocating for tariff exemptions on life-saving medical products.
Atopic dermatitis clinical trials are research studies aimed at evaluating the safety, effectiveness, and tolerability of new or existing treatments, including drugs, biologics, and topical therapies, for managing atopic dermatitis, a chronic inflammatory skin disorder. These trials are essential for advancing treatment options by measuring outcomes such as symptom relief, reduction in flare-ups, and improvements in patients’ quality of life.
The primary phases of atopic dermatitis clinical trials include Phase I, Phase II, Phase III, and Phase IV. Phase I trials represent the initial testing of a new treatment in humans, usually involving a small cohort of approximately 20-80 participants. These trials apply to both small and large molecules and use various study designs, including interventional and observational approaches. Trials can be conducted in-house or outsourced and cater to end users such as contract research organizations, pharmaceutical and biotechnology companies, and academic and research institutes.
The atopic dermatitis clinical trials market research report is one of a series of new reports that provides atopic dermatitis clinical trials market statistics, including atopic dermatitis clinical trials industry global market size, regional shares, competitors with a atopic dermatitis clinical trials market share, detailed atopic dermatitis clinical trials market segments, market trends and opportunities, and any further data you may need to thrive in the atopic dermatitis clinical trials industry. This atopic dermatitis clinical trials market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The atopic dermatitis clinical trials market consists of revenues earned by entities by providing services such as preclinical and translational research services, decentralized and virtual trial services, and patient engagement and retention programs. The market value includes the value of related goods sold by the service provider or included within the service offering. The atopic dermatitis clinical trials market also includes sales of topical corticosteroids, oral immunosuppressants, barrier repair creams, and antihistamines. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Atopic Dermatitis Clinical Trials Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on atopic dermatitis clinical trials market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for atopic dermatitis clinical trials? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The atopic dermatitis clinical trials market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Phase: Phase I; Phase II; Phase III; Phase IV2) By Molecule Type: Small Molecules; Large Molecules
3) By Study Designs: Interventional; Observational
4) By Mode: Outsourced; in-House
5) By Industry: Contract Research Organizations; Pharmaceutical and Biotechnology Companies; Research and Academic Institutes
Subsegments:
1) By Phase I: Safety and tolerability studies; Dose-Escalation studies; Pharmacokinetics (PK) and Pharmacodynamics (PD) Studies2) By Phase II: Dose-Ranging Studies; Efficacy and Safety Studies; Randomized Controlled Trials (RCTs); Biomarker Identification Studies
3) By Phase III: Large-Scale Efficacy Studies; Comparative Effectiveness Trials; Multicenter Randomized Trials; Long-Term Safety Assessment
4) By Phase IV: Post-Marketing Surveillance; Real-World Evidence Studies; Drug Utilization Studies; Health Outcomes and Quality of Life Studies
Companies Mentioned: Pfizer Inc.; Johnson & Johnson; AbbVie Inc.; Novartis International AG; Sanofi S.A.; GlaxoSmithKline Plc; Eli Lilly and Company; Amgen Inc.; Regeneron Pharmaceuticals Inc.; Organon & Co.; Charles River Laboratories International Inc.; LEO Pharma A/S; QIMA Limited; Medpace Holdings Inc.; Novotech Health Holdings Pte Ltd.; Biocytogen Pharmaceuticals Co. Ltd.; REPROCELL Inc.; AnaptysBio Inc.; Imavita S.A.S.; Oncodesign Services S.A.S.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Atopic Dermatitis Clinical Trials market report include:- Pfizer Inc.
- Johnson & Johnson
- AbbVie Inc.
- Novartis International AG
- Sanofi S.A.
- GlaxoSmithKline Plc
- Eli Lilly and Company
- Amgen Inc.
- Regeneron Pharmaceuticals Inc.
- Organon & Co.
- Charles River Laboratories International Inc.
- LEO Pharma A/S
- QIMA Limited
- Medpace Holdings Inc.
- Novotech Health Holdings Pte Ltd.
- Biocytogen Pharmaceuticals Co. Ltd.
- REPROCELL Inc.
- AnaptysBio Inc.
- Imavita S.A.S.
- Oncodesign Services S.A.S.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 2.74 Billion |
| Forecasted Market Value ( USD | $ 4.28 Billion |
| Compound Annual Growth Rate | 11.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |