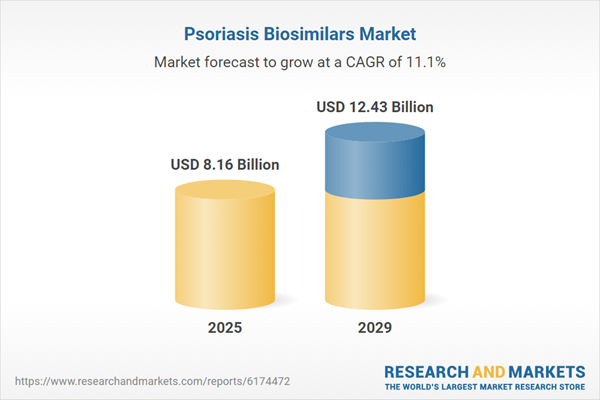
The psoriasis biosimilars market size is expected to see rapid growth in the next few years. It will grow to $12.43 billion in 2029 at a compound annual growth rate (CAGR) of 11.1%. The growth in the forecast period can be attributed to the rising global incidence of psoriasis, evolving switching policies and interchangeability regulations, expansion of biosimilar manufacturing, enhanced patient and physician education, and the adoption of bundled pricing and value-based contracts. Key trends in the forecast period include innovative biosimilar production platforms, AI-driven patient stratification, the launch of advanced drug delivery devices, development of next-generation formulations, and strategic collaborations within the industry.
The rising diagnosis rates of psoriatic arthritis are expected to drive growth in the psoriasis biosimilars market. Psoriatic arthritis is a chronic autoimmune condition that causes joint and skin inflammation, often affecting individuals with a history of psoriasis. Improved screening protocols have enabled earlier detection and more accurate diagnosis by identifying subtle joint symptoms that previously went unnoticed. Psoriasis biosimilars provide affordable alternatives to biologic therapies, effectively managing joint inflammation and skin symptoms. For instance, in May 2024, according to the National Institutes of Health (NIH), the prevalence of psoriatic arthritis in 2022 was 0.221%, with an incidence rate of 13.54 per 100,000 people. This increasing diagnosis trend is therefore fueling the psoriasis biosimilars market.
Companies in the market are focusing on innovative treatment solutions to improve patient outcomes and accessibility. Biosimilars for plaque psoriasis are lower-cost alternatives to approved biologics that manage chronic autoimmune skin conditions characterized by red, scaly patches. For example, in May 2025, Sandoz Group AG, a Switzerland-based pharmaceutical company, launched the Pyzchiva autoinjector, the first commercially available ustekinumab biosimilar autoinjector. Pyzchiva is approved for adults with plaque psoriasis, psoriatic arthritis, and Crohn’s disease, as well as pediatric patients aged six years and older (weighing over 60 kg) with plaque psoriasis. The autoinjector is designed for accurate automatic dosing, reduced injection pain, compact design, and flexible storage, enhancing patient adherence and quality of life.
In September 2023, Sandoz partnered with Samsung Bioepis, a South Korea-based biopharmaceutical company, to commercialize the SB17 (ustekinumab) biosimilar in Europe and North America. This collaboration aims to expand patient access to high-quality, cost-effective immunology treatments while strengthening Sandoz’s position in the global biosimilars market.
Major players in the psoriasis biosimilars market are Pfizer Inc., Merck & Co. Inc., Amgen Inc., Biogen Inc., Sandoz Group AG, Fresenius Kabi AG, Intas Pharmaceuticals Ltd., STADA Arzneimittel AG, Aurobindo Pharma Ltd., Dr. Reddy’s Laboratories Ltd., Cipla Ltd., Samsung Bioepis Co. Ltd., Lupin Ltd., Zydus Lifesciences Ltd., Celltrion Inc., Torrent Pharmaceuticals Ltd., Bio-Thera Solutions Ltd., Coherus BioSciences Inc., mAbxience S.A., Reliance Life Sciences Pvt. Ltd., and Alvotech hf.
North America was the largest region in the psoriasis biosimilars market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in this market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the psoriasis biosimilars market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the consequent trade frictions in spring 2025 are severely impacting the pharmaceutical companies contend with tariffs on APIs, glass vials, and lab equipment inputs with few alternative sources. Generic drug makers, operating on razor-thin margins, are especially vulnerable, with some reducing production of low-profit medicines. Biotech firms face delays in clinical trials due to tariff-related shortages of specialized reagents. In response, the industry is expanding API production in India and Europe, increasing inventory stockpiles, and pushing for trade exemptions for essential medicines.
Psoriasis biosimilars are biologic drugs engineered to closely replicate the structure, function, safety, and efficacy of approved reference biologics used for treating moderate to severe psoriasis. These biosimilars offer a more affordable option compared to original biologics, improving patient access to advanced treatments without compromising quality or clinical outcomes.
The priamry drug classes in psoriasis biosimilars include TNF-alpha inhibitors, infliximab, etanercept, adalimumab, and other biosimilars. TNF-alpha inhibitors are biologics that block tumor necrosis factor-alpha (TNF-α), a protein that drives inflammation in psoriasis. They are applied to various psoriasis types, including plaque, guttate, inverse, and pustular psoriasis, and administered via subcutaneous, intravenous, oral, or topical routes. Distribution channels include hospital pharmacies, retail pharmacies, and online pharmacies.
The psoriasis biosimilars market research report is one of a series of new reports that provides psoriasis biosimilars market statistics, including psoriasis biosimilars industry global market size, regional shares, competitors with a psoriasis biosimilars market share, detailed psoriasis biosimilars market segments, market trends and opportunities, and any further data you may need to thrive in the psoriasis biosimilars industry. This psoriasis biosimilars market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The psoriasis biosimilars market consists of sales of tumor necrosis factor inhibitors, monoclonal antibodies, subcutaneous and intravenous formulations, subcutaneous injections, and interleukin inhibitors. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Psoriasis Biosimilars Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on psoriasis biosimilars market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for psoriasis biosimilars? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The psoriasis biosimilars market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Drug Class: TNF-Alpha Inhibitors; Infliximab; Etanercept; Adalimumab; Other Biosimilars2) By Disease Type: Plaque Psoriasis; Guttate Psoriasis; Inverse Psoriasis; Pustular Psoriasis
3) By Route of Administration: Subcutaneous; Intravenous; Oral; Topical
4) By Distribution Channel: Hospital Pharmacies; Retail Pharmacies; Online Pharmacies
Subsegments:
1) By TNF-Alpha Inhibitors: Monoclonal Antibody Biosimilars; Fusion Protein Biosimilars2) By Infliximab: Remicade Biosimilars; CT-P13 Biosimilars
3) By Etanercept: Enbrel Biosimilars; SB4 Biosimilars
4) By Adalimumab: Humira Biosimilars; ABP 501 Biosimilars
5) By Other Biosimilars: Ustekinumab Biosimilars; Secukinumab Biosimilars
Companies Mentioned: Pfizer Inc.; Merck & Co. Inc.; Amgen Inc.; Biogen Inc.; Sandoz Group AG; Fresenius Kabi AG; Intas Pharmaceuticals Ltd.; STADA Arzneimittel AG; Aurobindo Pharma Ltd.; Dr. Reddy’s Laboratories Ltd.; Cipla Ltd.; Samsung Bioepis Co. Ltd.; Lupin Ltd.; Zydus Lifesciences Ltd.; Celltrion Inc.; Torrent Pharmaceuticals Ltd.; Bio-Thera Solutions Ltd.; Coherus BioSciences Inc.; mAbxience S.A.; Reliance Life Sciences Pvt. Ltd.; Alvotech hf.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Psoriasis Biosimilars market report include:- Pfizer Inc.
- Merck & Co. Inc.
- Amgen Inc.
- Biogen Inc.
- Sandoz Group AG
- Fresenius Kabi AG
- Intas Pharmaceuticals Ltd.
- STADA Arzneimittel AG
- Aurobindo Pharma Ltd.
- Dr. Reddy’s Laboratories Ltd.
- Cipla Ltd.
- Samsung Bioepis Co. Ltd.
- Lupin Ltd.
- Zydus Lifesciences Ltd.
- Celltrion Inc.
- Torrent Pharmaceuticals Ltd.
- Bio-Thera Solutions Ltd.
- Coherus BioSciences Inc.
- mAbxience S.A.
- Reliance Life Sciences Pvt. Ltd.
- Alvotech hf.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 8.16 Billion |
| Forecasted Market Value ( USD | $ 12.43 Billion |
| Compound Annual Growth Rate | 11.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 22 |