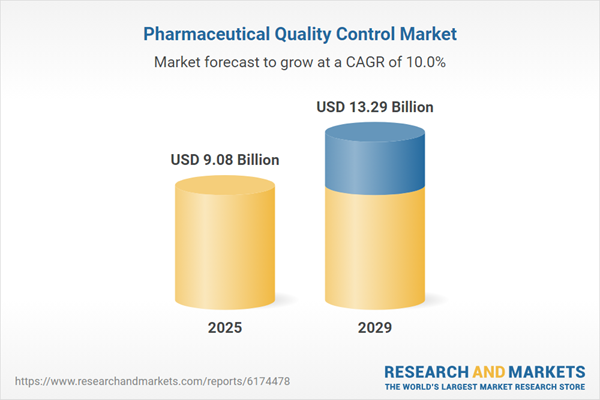
The pharmaceutical quality control market size is expected to see rapid growth in the next few years. It will grow to $13.29 billion in 2029 at a compound annual growth rate (CAGR) of 10%. The growth in the forecast period can be attributed to the rising adoption of artificial intelligence in quality control processes, an increasing need for precision medicine testing, growing demand for high-quality pharmaceuticals, expansion of contract manufacturing services, and a heightened focus on real-time quality monitoring systems. Key trends during this period include advancements in AI-powered visual inspection systems, innovative microbial detection technologies using biosensors, development of cloud-based quality control platforms, innovations in real-time environmental monitoring systems, and progress in digital twin technology for process simulation.
The rising demand for biosimilars is expected to drive growth in the pharmaceutical quality control market. Biosimilars are biologic medical products that are highly similar to an approved reference biologic, with no clinically meaningful differences in safety, purity, or potency. The demand for biosimilars is increasing due to patent expirations on original biologics, which allow more affordable alternatives to enter the market. Pharmaceutical quality control plays a critical role in ensuring that biosimilars meet strict safety, purity, and consistency standards by rigorously comparing them against reference biologics, supporting regulatory compliance and market approval. For example, Cardinal Health Inc., a US-based healthcare company, reported that the number of FDA-approved biosimilars in the US rose from 33 in January 2022 (with 21 commercially available) to 40 in 2023, with 25 commercially available. This trend highlights the growing need for quality control solutions in biosimilar production.
Companies in the pharmaceutical quality control market are increasingly adopting technological innovations such as AI-powered software platforms to improve testing accuracy, efficiency, and regulatory compliance. Intelligent software platforms use automation, data analytics, and artificial intelligence to detect anomalies, monitor real-time data, and streamline compliance throughout the pharmaceutical production process. For instance, in June 2025, Dycine Pharmaceuticals Ltd., an India-based company, launched an AI-driven quality control platform that leverages predictive analytics, automated reporting, and seamless integration with existing manufacturing systems to reduce errors, improve efficiency, and ensure consistent product quality across production batches.
In October 2024, the United States Pharmacopeial Convention (USP), a US-based independent scientific organization, acquired Stratix Labs, a provider of advanced microbiological testing solutions. This acquisition enables USP to expand its microbiological testing and contamination control capabilities, reduce microbial contamination risks, and address gaps in pharmaceutical contamination control strategies, thereby ensuring safer, higher-quality products reach patients.
Major players in the pharmaceutical quality control market are Thermo Fisher Scientific Inc., Abbott Laboratories, Danaher Corporation, Merck KGaA, Becton, Dickinson and Company, McKinsey And Company Inc., SGS S.A., Eurofins Scientific SE, WuXi AppTec Co. Ltd., Charles River Laboratories International Inc., PerkinElmer Inc., Mettler-Toledo International Inc., bioMérieux SA, Shimadzu Scientific Instruments Inc., Waters Corporation, Randox Laboratories Ltd., SOLVIAS AG, Esco Micro Pte. Ltd, REMI Elektrotechnik Limited, Lucideon Limited, and BRAM-COR S.p.A.
North America was the largest region in the pharmaceutical quality control market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in pharmaceutical quality control market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the pharmaceutical quality control market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the consequent trade frictions in spring 2025 are severely impacting the pharmaceutical companies contend with tariffs on APIs, glass vials, and lab equipment inputs with few alternative sources. Generic drug makers, operating on razor-thin margins, are especially vulnerable, with some reducing production of low-profit medicines. Biotech firms face delays in clinical trials due to tariff-related shortages of specialized reagents. In response, the industry is expanding API production in India and Europe, increasing inventory stockpiles, and pushing for trade exemptions for essential medicines.
Pharmaceutical quality control (QC) is a structured process designed to verify the identity, strength, purity, and consistency of pharmaceutical products. It involves thorough testing of raw materials, in-process samples, and finished products to ensure compliance with established standards, thereby guaranteeing the safety and efficacy of medicines before they reach patients.
The primary products in pharmaceutical quality control include consumables, services, and instruments. QC consumables consist of essential materials and supplies such as culture media, reagents, assay kits, filters, and pipette tips used during laboratory testing to maintain the quality, safety, and regulatory compliance of pharmaceutical products. These consumables can be deployed via cloud-based, web-based, or on-premises methods. The analytical processes include sterility testing, membrane filtration, direct inoculation, bioburden testing, aerobic and anaerobic count testing, spore count testing, fungi or mold count testing, endotoxin testing, and more. Pharmaceutical QC products and services are used by pharmaceutical and biotechnology companies, contract development and manufacturing organizations (CDMOs), contract research organizations (CROs), and research laboratories.
The pharmaceutical quality control market research report is one of a series of new reports that provides pharmaceutical quality control market statistics, including pharmaceutical quality control industry global market size, regional shares, competitors with a pharmaceutical quality control market share, detailed pharmaceutical quality control market segments, market trends and opportunities, and any further data you may need to thrive in the pharmaceutical quality control industry. This pharmaceutical quality control market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The pharmaceutical quality control market consists of revenues earned by entities by providing services such as analytical testing services, microbiological testing services, stability testing services, sterility assurance services, and validation and qualification services. The market value includes the value of related goods sold by the service provider or included within the service offering. The pharmaceutical quality control market also includes sales of reagents and assay kits, filters and membranes, sterility testing systems, and chromatography columns and consumables. Values in this market are ‘factory gate’ values; that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Pharmaceutical Quality Control Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on pharmaceutical quality control market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for pharmaceutical quality control? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The pharmaceutical quality control market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product: Consumables; Services; Instruments2) By Deployment Type: Cloud-Based and Web-Based; on-Premises
3) By Analysis Type: Sterility Testing; Membrane Filtration; Direct Inoculation; Bio Burden Testing; Aerobic Count Testing; Anaerobic Count Testing; Spore Count Testing; Fungi or Mold Count Testing; Endotoxin Testing; Other Analysis Types
4) By End-user: Pharmaceutical and Biotechnology Companies; Contract Development and Manufacturing Organization Or Contract Research Organization; Research Laboratories
Subsegments:
1) By Consumables: Culture Media; Reagents; Assay Kits; Filters and Membranes; Pipette Tips2) By Services: Microbiological Testing Services; Analytical Testing Services; Stability Testing Services; Sterility Assurance Services; Validation and Qualification Services
3) By Instruments: Chromatography Systems; Spectroscopy Instruments; Particle Size Analyzers; Potential of Hydrogen Meters and Electrical Conductivity Meters; Autoclaves and Incubators
Companies Mentioned: Thermo Fisher Scientific Inc.; Abbott Laboratories; Danaher Corporation; Merck KGaA; Becton, Dickinson and Company; McKinsey and Company Inc.; SGS S.A.; Eurofins Scientific SE; WuXi AppTec Co. Ltd.; Charles River Laboratories International Inc.; PerkinElmer Inc.; Mettler-Toledo International Inc.; bioMérieux SA; Shimadzu Scientific Instruments Inc.; Waters Corporation; Randox Laboratories Ltd.; SOLVIAS AG; Esco Micro Pte. Ltd; REMI Elektrotechnik Limited; Lucideon Limited; BRAM-COR S.p.A.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Pharmaceutical Quality Control market report include:- Thermo Fisher Scientific Inc.
- Abbott Laboratories
- Danaher Corporation
- Merck KGaA
- Becton, Dickinson and Company
- McKinsey And Company Inc.
- SGS S.A.
- Eurofins Scientific SE
- WuXi AppTec Co. Ltd.
- Charles River Laboratories International Inc.
- PerkinElmer Inc.
- Mettler-Toledo International Inc.
- bioMérieux SA
- Shimadzu Scientific Instruments Inc.
- Waters Corporation
- Randox Laboratories Ltd.
- SOLVIAS AG
- Esco Micro Pte. Ltd
- REMI Elektrotechnik Limited
- Lucideon Limited
- BRAM-COR S.p.A.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 9.08 Billion |
| Forecasted Market Value ( USD | $ 13.29 Billion |
| Compound Annual Growth Rate | 10.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 22 |