The expansion is driven by multiple factors, including a growing elderly population, the increasing incidence of pancreatic cancer, and advancements in diagnostic technologies that support early detection. As pancreatic cancer is often identified at an advanced stage due to its asymptomatic onset and deep anatomical positioning, there is a rising demand for precise, early-stage diagnostics. In the U.S., a range of diagnostic tools from imaging systems and blood-based biomarker tests to molecular platforms are playing a crucial role in confirming diagnoses, determining disease stage, and informing treatment paths. Demand continues to rise for non-invasive, high-accuracy solutions capable of addressing the challenges of late-stage diagnosis.
Technology is accelerating change across the diagnostic ecosystem, with AI-powered tools advancing imaging precision and real-time detection capabilities. Imaging platforms integrated with artificial intelligence are offering physicians earlier insights by interpreting scans faster and more accurately. These innovations are reducing diagnostic delays and improving the chances of detecting the disease at treatable stages, a vital component of successful outcomes in pancreatic cancer cases.
The instruments segment generated USD 404.5 million in 2024 and is projected to hit USD 811.9 million by 2034, growing at a CAGR of 7.3%. This segment serves as the foundation of the U.S. pancreatic cancer diagnostics market. It includes next-generation imaging equipment, endoscopy systems, and molecular diagnostic tools, all crucial for detecting and managing a disease that typically lacks early warning signs. Increasing deployment of AI-integrated technologies and advanced hardware in clinical settings is further propelling this segment forward as hospitals and imaging centers expand capacity and adopt cutting-edge systems.
In 2024, the blood test segment held a 30.2% share. Non-invasive blood-based diagnostics are gaining momentum due to their ease of use and potential for early-stage disease identification. Widely used biomarkers such as CEA and CA 19-9 remain standard for tracking disease progression, though limited sensitivity in initial stages has prompted the development of more advanced molecular diagnostics. Novel tests utilizing multiplex biomarkers or detecting electrical changes in plasma are currently emerging as promising additions to early screening protocols.
The diagnostic imaging centers segment held a 34.6% share in 2024. These facilities serve as essential access points for initial diagnostic evaluations due to their deployment of high-resolution imaging technologies capable of detecting abnormalities in pancreatic tissues. The increasing need for accurate and rapid diagnostics has led to expanded use of systems such as PCR platforms, NGS technologies, and liquid biopsy analyzers within imaging centers. These advanced solutions support the detection of genetic alterations, circulating tumor DNA, and specific biomarkers, reinforcing the role of imaging centers in timely cancer diagnosis.
Key industry players in the U.S. Pancreatic Cancer Diagnostic Market include Olympus, Illumina, Thermo Fisher Scientific, QIAGEN, Danaher, Canon, Siemens Healthineers, Myriad Genetics, Boston Scientific, Becton, Dickinson and Company, Agilent Technologies, Sysmex, Abbott Laboratories, GE Healthcare, Koninklijke Philips, and F. Hoffmann-La Roche. Companies in the U.S. pancreatic cancer diagnostic market are focusing on targeted innovation, strategic collaborations, and product diversification to maintain a competitive edge. Leading firms are investing in advanced AI-driven imaging systems and liquid biopsy technologies to improve early detection capabilities. Expanding R&D pipelines for next-generation biomarker panels and molecular diagnostics allows companies to address unmet clinical needs. Partnerships with academic institutions and diagnostic labs are accelerating product development and validation. Furthermore, firms are scaling their presence across diagnostic imaging centers and hospitals by enhancing system compatibility and integration
Comprehensive Market Analysis and Forecast
- Industry trends, key growth drivers, challenges, future opportunities, and regulatory landscape
- Competitive landscape with Porter’s Five Forces and PESTEL analysis
- Market size, segmentation, and regional forecasts
- In-depth company profiles, business strategies, financial insights, and SWOT analysis
This product will be delivered within 2-4 business days.
Table of Contents
Companies Mentioned
The companies profiled in this U.S. Pancreatic Cancer Diagnostic market report include:- Abbott Laboratories
- Agilent Technologies
- Becton, Dickinson and Company
- Boston Scientific
- Canon
- Danaher
- F Hoffmann-La Roche
- GE Healthcare
- Illumina
- Koninklijke Philips
- Myriad Genetics
- Olympus
- QIAGEN
- Siemens Healthineers
- Sysmex
- Thermo Fisher Scientific
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 60 |
| Published | September 2025 |
| Forecast Period | 2024 - 2034 |
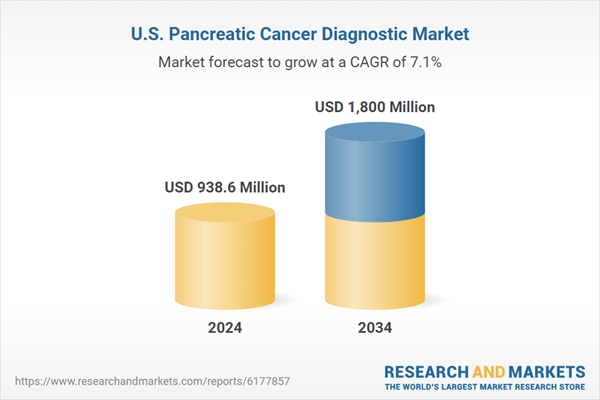
| Estimated Market Value ( USD | $ 938.6 Million |
| Forecasted Market Value ( USD | $ 1800 Million |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | United States |
| No. of Companies Mentioned | 17 |