Introduction of Non-Cancer Liquid Biopsy Testing Market
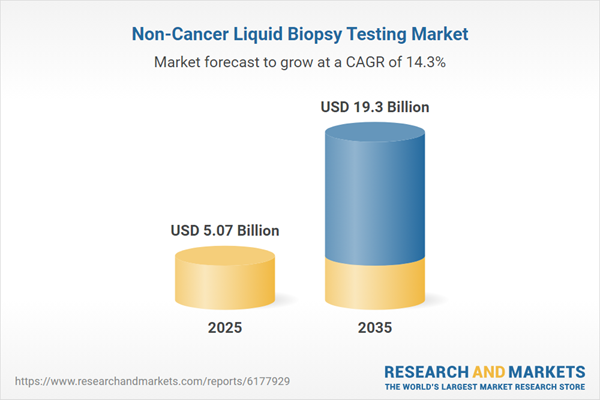
The global non-cancer liquid biopsy testing market, initially valued at $4.27 billion in 2024, is projected to witness substantial growth, surging to $19.30 billion by 2035, marking a remarkable compound annual growth rate (CAGR) of 14.30% over the period from 2025 to 2035.
The global non‑cancer liquid biopsy testing market is being propelled by the rising burden of chronic diseases, from cardiovascular and neurological disorders to prenatal and transplant care, and the growing demand for minimally invasive, real‑time diagnostics. By analyzing blood‑based biomarkers, liquid biopsy enables early detection and dynamic monitoring across a spectrum of indications without the risks of traditional sampling procedures. Advances in next‑generation sequencing and assay sensitivity, coupled with significant industry investment, have positioned liquid biopsy as the preferred approach for applications such as non‑invasive prenatal testing, graft‑rejection surveillance and infectious‑disease detection. However, high capital and per‑test costs, along with an uneven reimbursement environment, continue to challenge widespread adoption. Strategic localization of workflows in underserved regions, exemplified by in‑country deployments of CE‑marked platforms, offers a powerful opportunity to reduce turnaround times, lower costs and broaden access, paving the way for liquid biopsy to become a cornerstone of precision and preventive medicine worldwide.
Market Introduction
The global non-cancer liquid biopsy testing market is rapidly growing as biopharma and diagnostics companies extend minimally invasive, blood‑based molecular assays beyond oncology into critical areas such as prenatal screening, transplant surveillance and infectious‑disease detection. By leveraging cell‑free DNA, microRNAs and exosomes, liquid biopsy tests such as Illumina’s Verifi, Natera’s Panorama and Karius’ Spectrum, deliver early, real‑time insights that traditionally required risky tissue sampling, improving patient safety and operational efficiency. Ongoing assay innovations and novel biomarker validations, combined with strategic partnerships, and targeted acquisitions, are localizing advanced workflows and reducing turnaround times, costs and logistical hurdles. As healthcare systems worldwide embrace precision and preventive medicine, the convergence of enhanced performance, regulatory compliance and digital‑health integration positions non‑cancer liquid biopsy as a cornerstone of next‑generation diagnostics.
Industrial Impact
The non-cancer liquid biopsy testing market is witnessing rapid industrialization as key players leverage strategic alliances, targeted acquisitions, and significant funding to embed advanced, minimally invasive workflows within regional healthcare ecosystems. By partnering with digital‑health providers and local laboratories, companies are streamlining end‑to‑end diagnostic pathways, slashing turnaround times and reducing per‑test costs without compromising sample integrity. Simultaneously, mergers and geographic expansions are strengthening in‑country capacities, while portfolio enhancements and new product launches are broadening application scopes, from prenatal and transplant monitoring to infectious‑disease detection. Substantial capital inflows have accelerated R&D and commercialization, enabling assay standardization and regulatory compliance at scale. Together, these industrial dynamics are transforming non‑cancer liquid biopsy from a niche innovation into a robust, globally accessible platform for precision and preventive medicine.
Market Segmentation:
Segmentation 1: by Application
- Prenatal Testing
- Organ Transplantation
- Others
Prenatal Testing Segment to Dominate the Non-Cancer Liquid Biopsy Testing Market (by Application)
The global non-cancer liquid biopsy testing market (by application) has been dominated by prenatal testing, representing 79.70% of the market share in 2024, and is expected to maintain its dominance during the forecast period. This reflects prenatal testing’s minimally invasive, single-blood-draw protocol with no risk, broad reimbursement coverage and universal guideline endorsements. These factors collectively support the growth of the prenatal testing in the non-cancer liquid biopsy space
Segmentation 2: Technology
- NGS
- PCR
- Others
NGS to Dominate the Non-Cancer Liquid Biopsy Testing Market (by Technology)
The global non-cancer liquid biopsy testing market (by technology) has been dominated by NGS, representing 59.68% of the market share in 2024, and is expected to maintain its dominance during the forecast period due to its unparalleled capacity for comprehensive, high-throughput genomic profiling in a single assay and its steadily declining per-sample costs enabled by technological advances and scale efficiencies.
Segmentation 3: by End User
- Clinical
- Research
Clinical to Dominate the Non-Cancer Liquid Biopsy Testing Market (by End User)
The global non-cancer liquid biopsy testing market (by end user) has been dominated by clinical segment, representing 86.22% of the market share in 2024, and is expected to maintain its dominance during the forecast period driven by rapid adoption of liquid biopsy in clinical laboratories and increasing utilization by pharmaceutical and biotech companies for drug development and clinical trial monitoring. This dominance reflects strong reimbursement pathways, integration into routine patient care and the escalating demand for minimally invasive diagnostics in both clinical practice and therapeutic innovation.
Segmentation 4: by Region
- North America
- U.S.
- Canada
- Europe
- U.K.
- France
- Germany
- Italy
- Spain
- Rest-of-Europe
- Asia-Pacific
- Japan
- China
- India
- Australia
- Rest-of-Asia-Pacific
- Latin America
- Brazil
- Mexico
- Rest-of-Latin America
- Middle East and Africa
- U.A.E.
- K.S.A.
- South Africa
- Rest-of-Middle East and Africa
The non-cancer liquid biopsy testing market in the Asia-Pacific region is expected to witness a significant growth rate of 15.77% during the forecast period.
Recent Developments
- In May 2025, BillionToOne, Inc. launched an expanded UNITY Fetal Risk Screen to assess fetal risk for up to 14 genetic conditions from a single blood sample as early as nine weeks into pregnancy.
- In Febrauary 2025, Yourgene Health launched IONA Care+, a comprehensive non-invasive prenatal testing (NIPT) service in the U.K. This service utilizes the IONA Nx NIPT Workflow to provide accurate screening for genetic conditions, including microdeletions.
- In Febrauary 2025, Acrannolife Genomics partnered with GOQii to launch a program aimed at enhancing post-transplant care in India through advanced digital and genomic solutions.
Demand - Drivers, Challenges, and Opportunities
Market Drivers:
Growing Disease Burden and Screening: The global non-cancer liquid biopsy testing market is witnessing growth due to the rising burden of chronic diseases and the need for non-invasive diagnostic tools. Liquid biopsy enables early detection and monitoring of conditions like cardiovascular and neurological disorders by analyzing biomarkers such as cfDNA and circulating/exosomal miRNAs. It offers real-time disease insights, particularly where traditional tissue biopsies are difficult. Beyond heart and brain health, it also supports applications in organ transplantation, prenatal testing, and infectious disease management.
Market Challenges:
Uncertain Reimbursement Landscape and High Setup Costs Hindering Widespread Adoption: The adoption of non-cancer liquid biopsy tests is limited by an uncertain and inconsistent reimbursement landscape, especially outside oncology applications. Despite their clinical potential, payers demand stronger evidence of cost-effectiveness, and rapid technological advancements often outpace policy updates. High setup costs for advanced sequencing platforms and expensive per-test pricing further restrict access, especially without insurance coverage. These challenges hinder widespread clinical integration, particularly in prenatal, infectious disease, and transplant-related diagnostics.
Market Opportunities:
Expanding Access in Underserved Regions: Non-cancer liquid biopsy companies are localizing advanced diagnostic services in underserved regions to improve access and reduce reliance on overseas labs. By implementing in-house workflows, such as Yourgene Health’s NIPT solutions in Morocco and Colombia, they’ve reduced turnaround times, costs, and test failures. These efforts enable faster, more accurate diagnoses while enhancing clinical autonomy. This approach presents a scalable opportunity for wider adoption across prenatal, transplant, and infectious disease diagnostics, promoting health equity and deeper market penetration.
How can this report add value to an organization?
Product/Innovation Strategy: The global non-cancer liquid biopsy testing market has been extensively segmented based on various categories, such as application, technology, end user, and region. This can help readers get a clear overview of which segments account for the largest share and which ones are well-positioned to grow in the coming years.
Growth/Marketing Strategy: Partnerships accounted for the maximum number of key developments, i.e., nearly 40% of the total developments in the global non-cancer liquid biopsy testing market were between January 2022 and May 2025.
Competitive Strategy: The global non-cancer liquid biopsy testing market has numerous established players with product portfolios. Key players in the global non-cancer liquid biopsy testing market analyzed and profiled in the study include established players offering products for non-cancer liquid biopsy testing systems.
Methodology
Key Considerations and Assumptions in Market Engineering and Validation
- The base year considered for the calculation of the market size is 2024. A historical year analysis has been done for the period FY2022-FY2023. The market size has been estimated for FY2024 and projected for the period FY2025-FY2035.
- The scope of this report has been carefully derived based on interactions with experts in different companies across the world.
- The market contribution of non-cancer liquid biopsy testing is anticipated to be launched in the future and has been calculated based on the historical analysis of the solutions.
- Revenues of the companies have been referenced from their annual reports for FY2023 and FY2024. For private companies, revenues have been estimated based on factors such as inputs obtained from primary research, funding history, market collaborations, and operational history.
- The market has been mapped based on the available non-cancer liquid biopsy tests. All the key companies with significant offerings in this field have been considered and profiled in this report.
Primary Research:
The primary sources involve industry experts in non-cancer liquid biopsy testing, including the market players offering products and services. Resources such as CEOs, vice presidents, marketing directors, and technology and innovation directors have been interviewed to obtain and verify both qualitative and quantitative aspects of this research study.
The key data points taken from the primary sources include:
- Validation and triangulation of all the numbers and graphs
- Validation of the report’s segmentation and key qualitative findings
- Understanding the competitive landscape and business model
- Current and proposed production values of a product by market players
- Validation of the numbers of the different segments of the market in focus
- Percentage split of individual markets for regional analysis
Secondary Research
Open Sources
- Certified publications, articles from recognized authors, white papers, directories, and major databases, among others
- Annual reports, SEC filings, and investor presentations of the leading market players
- Company websites and detailed study of their product portfolio
- Gold standard magazines, journals, white papers, press releases, and news articles
- Paid databases
The key data points taken from the secondary sources include:
- Segmentations and percentage shares
- Data for market value
- Key industry trends of the top players in the market
- Qualitative insights into various aspects of the market, key trends, and emerging areas of innovation
- Quantitative data for mathematical and statistical calculations
Key Market Players and Competition Synopsis
The companies profiled have been selected based on inputs gathered from an analysis of company coverage, product portfolio, and market penetration.
Some prominent names established in this market are:
- AcrannoLife Genomics
- Agilent Technologies, Inc.
- BillionToOne Inc.
- BGI Group
- CareDx Inc.
- Eurofins Scientific SE
- F. Hoffmann-La Roche Ltd.
- Illumina, Inc.
- Karius, Inc.
- Laboratory Corporation of America Holdings
- Lilac Insights Pvt. Ltd.
- MedGenome Labs Ltd.
- Natera, Inc.
- Yourgene Health (Novacyt Group)
- Oncocyte Corporation
Table of Contents
Companies Mentioned
- AcrannoLife Genomics
- Agilent Technologies, Inc.
- BillionToOne Inc.
- BGI Group
- CareDx Inc.
- Eurofins Scientific SE
- F. Hoffmann-La Roche Ltd.
- Illumina, Inc.
- Karius, Inc.
- Laboratory Corporation of America Holdings
- Lilac Insights Pvt. Ltd.
- MedGenome Labs Ltd.
- Natera, Inc.
- Yourgene Health (Novacyt Group)
- Oncocyte Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 146 |
| Published | October 2025 |
| Forecast Period | 2025 - 2035 |
| Estimated Market Value ( USD | $ 5.07 Billion |
| Forecasted Market Value ( USD | $ 19.3 Billion |
| Compound Annual Growth Rate | 14.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |