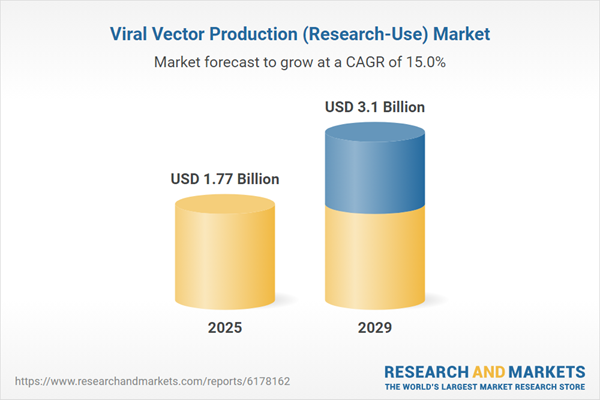
The viral vector production (research-use) market size is expected to see rapid growth in the next few years. It will grow to $3.1 billion in 2029 at a compound annual growth rate (CAGR) of 15%. The growth during the forecast period is driven by the rising prevalence of genetic disorders, the expansion of the preclinical gene therapy pipeline, the growing role in regenerative medicine studies, increasing research on neurodegenerative diseases, and higher funding for rare disease research. Key trends in the forecast period include advancements in gene therapy research, automation in vector quality control (QC), development of hybrid and chimeric vectors, automation across upstream and downstream processes, and synthetic biology-based vector modifications.
The increasing frequency of genetic disorders is anticipated to drive the growth of the viral vector production (research-use) market in the coming years. Genetic disorders are medical conditions caused by abnormalities in an individual's deoxyribonucleic acid, which may be inherited from one or both parents or arise from new genetic mutations. This rise in genetic disorders is linked to advanced parental age, which heightens the chances of passing genetic mutations to offspring. As the number of genetic disorders grows, there is a rising need for advanced gene delivery methods to explore disease mechanisms and develop possible treatments, thereby supporting the expansion of viral vector production for research purposes. For example, in November 2024, the Centers for Disease Control and Prevention, a public health agency based in the United States, reported that Trisomy 21 occurs in approximately one in 643 births, resulting in nearly 5713 new cases each year, while Trisomy 18 occurs in about one in 3336 births, equating to around 1101 cases annually. Consequently, the increasing prevalence of genetic disorders is expected to support the growth of the viral vector production (research-use) market.
Leading companies in the viral vector production market are concentrating on creating advanced solutions such as specialized cell culture media to enhance transfection efficiency, boost viral vector output, and maintain consistent quality for research use. Cell culture media are nutrient-enriched solutions formulated to sustain and grow cells outside their natural settings. These media contain critical elements such as amino acids, vitamins, and growth factors, tailored for particular cell types and research needs. For instance, in January 2023, FUJIFILM Irvine Scientific, a life sciences company based in the United States, launched BalanCD HEK293 Viral Feed, the first chemically defined nutrient feed specifically optimized for HEK293 suspension cultures used in adeno-associated virus viral vector production. This product aims to significantly improve viral yields, offering up to a 67 percent increase in viral packaging efficiency compared to basic media. It is intended to support scalable gene therapy and biotechnology processes, ensuring consistent performance across formats and compatibility with a variety of media systems.
In January 2024, Oxford Biomedica Plc, a gene and cell therapy company based in the United Kingdom, acquired ABL Europe for an undisclosed sum. The acquisition is intended to broaden Oxford Biomedica's footprint in the European market and enhance its contract development and manufacturing organization services, especially in viral vector production. ABL Europe is a company located in France that focuses on the development and manufacture of viral vectors.
Major players in the viral vector production (research-use) market are Sanofi S.A., Thermo Fisher Scientific Inc., Merck KGaA, Becton, Dickinson and Company, Lonza Group AG, Sartorius AG, Charles River Laboratories International Inc., FUJIFILM Diosynth Biotechnologies U.S.A. Inc., Curia Inc., AGC Biologics GmbH, SK pharmteco Inc., Oxford Biomedica Plc, Waisman Biomanufacturing, Voyager Therapeutics Inc., FinVector Oy, Batavia Biosciences BV., GENEZEN LABORATORIES, BioNTech IMFS GmbH, VGXI Inc., Tonix Pharmaceuticals, Cell and Gene Therapy Catapult, and Mustang Bio Inc.
North America was the largest region in the viral vector production (research-use) market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in viral vector production (research-use) report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the viral vector production (research-use) market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the consequent trade frictions in spring 2025 are severely impacting the pharmaceutical companies contend with tariffs on APIs, glass vials, and lab equipment inputs with few alternative sources. Generic drug makers, operating on razor-thin margins, are especially vulnerable, with some reducing production of low-profit medicines. Biotech firms face delays in clinical trials due to tariff-related shortages of specialized reagents. In response, the industry is expanding API production in India and Europe, increasing inventory stockpiles, and pushing for trade exemptions for essential medicines.
Viral vector production (research-use) refers to the laboratory process of creating modified viruses capable of efficiently delivering genetic material into target cells for experimental applications. These vectors are engineered to be replication-deficient and safe for use in controlled research settings. The process includes vector design, production in host cells, and purification to ensure quality, safety, and effectiveness in scientific research.
The primary types of viral vector production (research-use) include adeno-associated virus (AAV), lentivirus, adenovirus, retrovirus, and others. Adeno-associated viruses are small viruses from the Parvoviridae family that deliver genetic material to cells but cannot replicate independently, relying instead on helper viruses. Production methods include transient transfection, stable cell lines, viral packaging systems, and others, carried out through workflows such as upstream processing and downstream processing. Applications include gene therapy, vaccines, oncology, infectious diseases, and others, with end users such as pharmaceutical and biopharmaceutical companies as well as research institutes.
The viral vector production (research-use) market research report is one of a series of new reports that provides viral vector production (research-use) market statistics, including viral vector production (research-use) industry global market size, regional shares, competitors with a viral vector production (research-use) market share, detailed viral vector production (research-use) market segments, market trends and opportunities, and any further data you may need to thrive in the viral vector production (research-use) industry. The viral vector production (research-use) market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The viral vector production (research-use) market consists of sales of baculovirus, herpes simplex virus (HSV), vaccinia virus, vesicular stomatitis virus (VSV), hybrid viral vectors, and measles virus vectors. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values and are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Viral Vector Production (Research-Use) Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on viral vector production (research-use) market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for viral vector production (research-use)? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The viral vector production (research-use) market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Report Scope
Markets Covered:
1) By Type: Adeno-Associated Virus (AAV); Lentivirus; Adenovirus; Retrovirus; Other Types2) By Production: Transient Transfection; Stable Cell Line; Viral Packaging System; Other Production Methods
3) By Workflow: Upstream Processing; Downstream Processing
4) By Application: Gene Therapy; Vaccines; Oncology; Infectious Diseases; Other Applications
5) By End-Use: Pharmaceutical And Biopharmaceutical Companies; Research Institutes
Subsegments:
1) By Adeno-Associated Virus: Single-Stranded Adeno-Associated Virus; Self-Complementary Adeno-Associated Virus; Hybrid Adeno-Associated Virus2) By Lentivirus: Human Immunodeficiency Virus-Based Lentivirus; Feline Immunodeficiency Virus-Based Lentivirus; Equine Infectious Anemia Virus-Based Lentivirus
3) By Adenovirus: Replication-Competent Adenovirus; Replication-Deficient Adenovirus; Helper-Dependent Adenovirus
4) By Retrovirus: Gamma Retrovirus; Spumavirus (Foamy Virus); Alpha Retrovirus
5) By Other Types: Herpes Simplex Virus Vectors; Baculovirus Vectors; Sendai Virus Vectors
Companies Mentioned: Sanofi S.A.; Thermo Fisher Scientific Inc.; Merck KGaA; Becton, Dickinson and Company; Lonza Group AG; Sartorius AG; Charles River Laboratories International Inc.; FUJIFILM Diosynth Biotechnologies U.S.A. Inc.; Curia Inc.; AGC Biologics GmbH; SK pharmteco Inc.; Oxford Biomedica Plc; Waisman Biomanufacturing; Voyager Therapeutics Inc.; FinVector Oy; Batavia Biosciences BV.; GENEZEN LABORATORIES; BioNTech IMFS GmbH; VGXI Inc.; Tonix Pharmaceuticals; Cell and Gene Therapy Catapult; Mustang Bio Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Viral Vector Production (Research-Use) market report include:- Sanofi S.A.
- Thermo Fisher Scientific Inc.
- Merck KGaA
- Becton, Dickinson and Company
- Lonza Group AG
- Sartorius AG
- Charles River Laboratories International Inc.
- FUJIFILM Diosynth Biotechnologies U.S.A. Inc.
- Curia Inc.
- AGC Biologics GmbH
- SK pharmteco Inc.
- Oxford Biomedica Plc
- Waisman Biomanufacturing
- Voyager Therapeutics Inc.
- FinVector Oy
- Batavia Biosciences BV.
- GENEZEN LABORATORIES
- BioNTech IMFS GmbH
- VGXI Inc.
- Tonix Pharmaceuticals
- Cell and Gene Therapy Catapult
- Mustang Bio Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | October 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.77 Billion |
| Forecasted Market Value ( USD | $ 3.1 Billion |
| Compound Annual Growth Rate | 15.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 23 |