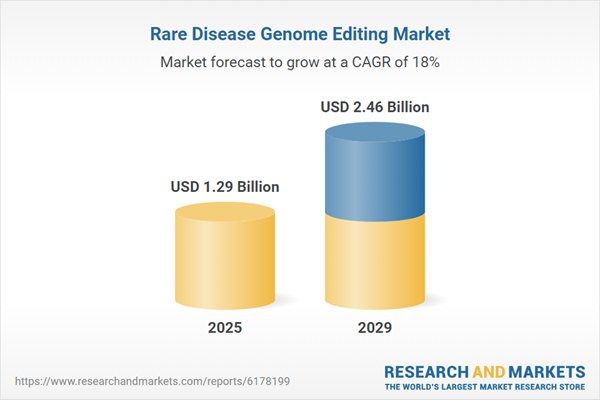
The rare disease genome editing market size is expected to see rapid growth in the next few years. It will grow to $2.46 billion in 2029 at a compound annual growth rate (CAGR) of 17.6%. The growth during the forecast period is fueled by increasing integration of artificial intelligence in genome editing, rising demand for targeted therapies, advancements in gene delivery systems, growing prevalence of multi-gene disorders, and heightened focus on pediatric rare diseases. The primary trends in the forecast period include development of multi-gene editing techniques, integration of AI-driven genomic analysis, technology-enabled personalized gene therapies, innovations in in vivo gene delivery systems, and advancements in high-throughput genome screening.
The growing emphasis on precision medicine is expected to drive the expansion of the rare disease genome editing market. Precision medicine involves tailoring prevention, diagnosis, and treatment strategies based on an individual’s genetic makeup, environment, and lifestyle. The increasing focus on this approach is fueled by advances in genomic sequencing and biomarker identification, which enable more accurate detection and targeted therapies. The rare disease genome editing market plays a key role in precision medicine by enabling the precise correction of genetic mutations, making treatments more personalized and effective. This approach addresses the root causes of rare disorders, improving patient outcomes and reducing reliance on symptom-based treatments. For example, in March 2024, Novotech, an Australia-based biotechnology company, reported that 43% of the 217 FDA-approved oncology therapies in 2023 were precision oncology treatments, with 78 of these incorporating DNA or NGS-detectable biomarkers. This growing focus on precision medicine is contributing to the growth of the rare disease genome editing market.
Companies in the rare disease genome editing market are advancing therapeutic platforms, particularly CRISPR-based gene editing systems, to enhance treatment precision and improve outcomes for previously untreatable genetic diseases. CRISPR-based gene editing systems are cutting-edge technologies that allow for precise alterations to the genome, enabling the correction of genetic defects or the introduction of therapeutic genes into cells. For example, in December 2023, Vertex Pharmaceuticals Inc., a US-based biopharmaceutical company, partnered with CRISPR Therapeutics Inc. and received conditional marketing authorization from the UK Medicines and Healthcare products Regulatory Agency (MHRA) for CASGEVY (exagamglogene autotemcel, exa-cel). CASGEVY is indicated for patients aged 12 and older with sickle cell disease (SCD) and recurrent vaso-occlusive crises or transfusion-dependent beta-thalassemia (TDT). The treatment works by editing a patient’s hematopoietic stem cells to reactivate fetal hemoglobin production, potentially reducing or eliminating disease symptoms and offering a functional cure for these conditions.
In November 2022, Alexion Pharmaceuticals Inc., a subsidiary of AstraZeneca, acquired LogicBio Therapeutics Inc. for an undisclosed amount. This acquisition allows Alexion to expand its presence in the field of rare disease genomics and leverage LogicBio's GeneRide platform to develop innovative therapies. LogicBio Therapeutics is a clinical-stage company specializing in genome-editing therapies for rare genetic disorders.
Major players in the rare disease genome editing market are Regeneron Pharmaceuticals Inc., Sarepta Therapeutics Inc., Ultragenyx Pharmaceutical Inc., Beam Therapeutics Inc., CRISPR Therapeutics AG, Rocket Pharmaceuticals Inc., Sangamo Therapeutics Inc., Arcturus Therapeutics Holdings Inc., Editas Medicine Inc., Krystal Biotech Inc., Caribou Biosciences Inc, Bluebird Bio Inc., SpliceBio S.L., Cellectis SA, Generation Bio Co., iECURE Inc., Affinia Therapeutics Inc., Precision Biosciences Inc., Intellia Therapeutics Inc., and MeiraGTx Holdings plc.
North America was the largest region in the rare disease genome editing market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in rare disease genome editing report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the rare disease genome editing market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the consequent trade frictions in spring 2025 are severely impacting the pharmaceutical companies contend with tariffs on APIs, glass vials, and lab equipment inputs with few alternative sources. Generic drug makers, operating on razor-thin margins, are especially vulnerable, with some reducing production of low-profit medicines. Biotech firms face delays in clinical trials due to tariff-related shortages of specialized reagents. In response, the industry is expanding API production in India and Europe, increasing inventory stockpiles, and pushing for trade exemptions for essential medicines.
Rare disease genome editing refers to the application of precise genetic modification technologies to correct or alter disease-causing mutations in patients with rare genetic disorders. This approach restores normal gene function, prevents disease progression, and enables the development of personalized therapeutic solutions for conditions with limited or no treatment options. It targets the root cause of the disease at the DNA level rather than managing symptoms.
The primary technologies used in rare disease genome editing include CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats with CRISPR-associated protein 9), transcription activator-like effector nucleases (TALEN), zinc finger nucleases (ZFN), and others. CRISPR-Cas9 employs a guide RNA and the Cas9 enzyme to precisely cut and modify DNA for therapeutic and research purposes. These technologies are applied to various disease types, including monogenic disorders, multifactorial disorders, and chromosomal disorders, and are used in therapeutic development and research by biotechnology and pharmaceutical companies, academic and government research institutes, and hospitals.
The rare disease genome editing market research report is one of a series of new reports that provides rare disease genome editing market statistics, including rare disease genome editing industry global market size, regional shares, competitors with a rare disease genome editing market share, detailed rare disease genome editing market segments, market trends and opportunities, and any further data you may need to thrive in the rare disease genome editing industry. This rare disease genome editing market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.`
The rare disease genome editing market consists of revenues earned by entities by providing services such as gene editing therapy development, genetic testing and diagnostics, preclinical research and validation, clinical trial management, and regulatory consulting for genome-based therapeutics. The market value includes the value of related goods sold by the service provider or included within the service offering. The rare disease genome editing market also includes sales of zinc finger nucleases, delivery vectors, modified cells, and therapeutic genome-editing formulations. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values and are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Rare Disease Genome Editing Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on rare disease genome editing market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for rare disease genome editing? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The rare disease genome editing market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Report Scope
Markets Covered:
1) By Technology: Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) Or CRISPR-Associated Protein 9; Transcription Activator-Like Effector Nucleases (TALEN); Zinc Finger Nucleases (ZFN); Other Technologies2) By Disease Type: Monogenic Disorders; Multifactorial Disorders; Chromosomal Disorders
3) By Application: Therapeutics; Research
4) By End User: Biotechnology And Pharmaceutical Companies; Academic And Government Research Institutes; Hospitals
Subsegments:
1) By Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) Or CRISPR-Associated Protein 9: Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-Associated Protein 9; Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-Associated Protein 12; Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-Associated Protein 13; Base Editing; Prime Editing2) By Transcription Activator-Like Effector Nucleases (TALEN): Standard Transcription Activator-Like Effector Nucleases (TALEN); Designer Transcription Activator-Like Effector Nucleases (dTALEN); Transcription Activator-Like Effector Nucleases (TALEN) Paired Nucleases
3) By Zinc Finger Nucleases (ZFN): Standard Zinc Finger Nucleases (ZFN); Engineered Zinc Finger Nucleases (ZFN); Modular Zinc Finger Nucleases (ZFN)
4) By Other Technologies: Meganucleases; Ribonucleic Acid (RNA)-Guided Nucleases; Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-Associated Transposases
Companies Mentioned: Regeneron Pharmaceuticals Inc.; Sarepta Therapeutics Inc.; Ultragenyx Pharmaceutical Inc.; Beam Therapeutics Inc.; CRISPR Therapeutics AG; Rocket Pharmaceuticals Inc.; Sangamo Therapeutics Inc.; Arcturus Therapeutics Holdings Inc.; Editas Medicine Inc.; Krystal Biotech Inc.; Caribou Biosciences Inc; Bluebird Bio Inc.; SpliceBio S.L.; Cellectis SA; Generation Bio Co.; iECURE Inc.; Affinia Therapeutics Inc.; Precision Biosciences Inc.; Intellia Therapeutics Inc.; MeiraGTx Holdings plc
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Rare Disease Genome Editing market report include:- Regeneron Pharmaceuticals Inc.
- Sarepta Therapeutics Inc.
- Ultragenyx Pharmaceutical Inc.
- Beam Therapeutics Inc.
- CRISPR Therapeutics AG
- Rocket Pharmaceuticals Inc.
- Sangamo Therapeutics Inc.
- Arcturus Therapeutics Holdings Inc.
- Editas Medicine Inc.
- Krystal Biotech Inc.
- Caribou Biosciences Inc
- Bluebird Bio Inc.
- SpliceBio S.L.
- Cellectis SA
- Generation Bio Co.
- iECURE Inc.
- Affinia Therapeutics Inc.
- Precision Biosciences Inc.
- Intellia Therapeutics Inc.
- MeiraGTx Holdings plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | October 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.29 Billion |
| Forecasted Market Value ( USD | $ 2.46 Billion |
| Compound Annual Growth Rate | 18.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |