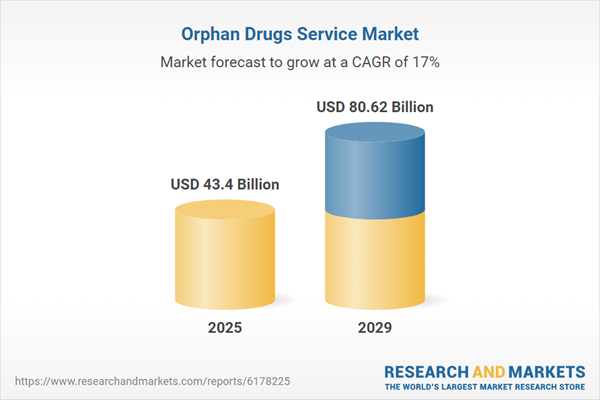
The orphan drugs service market size has grown rapidly in recent years. It will grow from $37.05 billion in 2024 to $43.4 billion in 2025 at a compound annual growth rate (CAGR) of 17.1%. The growth during the historic period was driven by increasing prevalence of rare diseases, rising government support, expanding patient advocacy initiatives, growing focus on unmet medical needs, and increasing collaboration between healthcare providers and researchers.
The orphan drugs service market size is expected to see rapid growth in the next few years. It will grow to $80.62 billion in 2029 at a compound annual growth rate (CAGR) of 16.7%. The growth during the forecast period is fueled by rising investment in biopharmaceutical research, growing demand for precision medicine, expanding adoption of advanced clinical trial designs, increasing venture capital funding, and greater emphasis on global regulatory harmonization. The primary trends in the forecast period include advancements in drug discovery platforms, innovation in cell and gene therapy services, integration of artificial intelligence in clinical trials, developments in biomarker-based patient selection, and adoption of digital health solutions for patient monitoring.
The increasing investment in rare diseases is expected to drive the growth of the orphan drugs service market in the coming years. Rare diseases are health conditions that affect a small portion of the population, typically fewer than 1 in 2,000 people. These conditions are often chronic, progressive, and can be life-threatening or disabling. The rise in investment is primarily driven by the growing recognition of unmet medical needs and the potential for substantial returns, supported by regulatory incentives such as orphan drug designation, market exclusivity, and lower development costs. Increased investment enhances orphan drug services by providing additional funding for research and development, enabling the creation of therapies for previously overlooked conditions. It accelerates the availability of drugs by supporting clinical trials, regulatory approvals, and improving patient access, thereby expanding treatment options for those with rare diseases. For example, in April 2024, Global Genes, a US-based nonprofit organization, reported that companies focused on rare disease drugs raised $7.1 billion in the first quarter of 2024, a significant 307% increase from the $1.8 billion raised during the same period in 2023. As a result, the growing investment in rare diseases is fueling the expansion of the orphan drugs service market.
Companies in the orphan drugs service market are increasingly focused on obtaining regulatory approvals to expedite the development and availability of treatments for rare diseases. Regulatory approvals are essential for legal testing, manufacturing, and marketing of drugs and treatments. For example, in February 2023, Askbio Inc., a US-based gene therapy company, received orphan drug designation from the European Commission for AB-1003, a novel gene therapy for limb-girdle muscular dystrophy. This adeno-associated virus (AAV)-based therapy aims to restore FKRP enzyme activity in muscle cells through a single intravenous infusion. AB-1003 has also been granted orphan drug status by the U.S. Food and Drug Administration (FDA), along with rare pediatric disease and fast track designations, underscoring the critical unmet need. The therapy is currently undergoing a Phase 1/2 clinical trial (LION-CS101) to evaluate its safety and efficacy in adults with genetically confirmed limb-girdle muscular dystrophy type 2I/R9 (LGMD2I/R9).
In April 2025, Norgine B.V., a Netherlands-based pharmaceutical company, acquired Theravia Pharma for an undisclosed amount. This acquisition allows Norgine to strengthen its rare disease portfolio and accelerate its growth in the rare and specialty pharmaceuticals sector. By expanding its range of life-changing therapies for patients with high unmet medical needs in Europe and the Asia-Pacific region (ANZ), Norgine aims to become a leading partner in rare diseases. Theravia Pharma, based in France, specializes in rare diseases and orphan drug services.
Major players in the orphan drugs service market are Johnson & Johnson Services Inc., F. Hoffmann-La Roche Ltd., Pfizer Inc., AbbVie Inc., Bayer AG, Sanofi S.A., Novartis AG, AstraZeneca PLC, The Bristol-Myers Squibb Company, GSK plc., Eli Lilly and Company, Takeda Pharmaceutical Company Limited, Amgen Inc., IQVIA Inc., CSL Behring GmbH, Lonza Group AG, BioMarin Pharmaceutical Inc., Fortrea Holdings Inc., Medpace Inc., Sarepta Therapeutics, Caidya Inc., and Cevidra Inc.
North America was the largest region in the orphan drugs service market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in orphan drugs service report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the orphan drugs service market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the consequent trade frictions in spring 2025 are severely impacting the pharmaceutical companies contend with tariffs on APIs, glass vials, and lab equipment inputs with few alternative sources. Generic drug makers, operating on razor-thin margins, are especially vulnerable, with some reducing production of low-profit medicines. Biotech firms face delays in clinical trials due to tariff-related shortages of specialized reagents. In response, the industry is expanding API production in India and Europe, increasing inventory stockpiles, and pushing for trade exemptions for essential medicines.
Orphan drug services refer to specialized healthcare and pharmaceutical support focused on facilitating the research, development, approval, and accessibility of medications for rare diseases, which affect a small and often underserved portion of the population. These services play a critical role in navigating complex regulatory and development processes, ensuring that essential therapies reach patients who have limited or no alternative treatment options.
The primary types of orphan drug services include congenital disease, genetic disease, tumors and cancer, chronic conditions, and others. Congenital diseases refer to medical conditions present from birth, caused by genetic abnormalities, developmental issues during pregnancy, or environmental factors affecting fetal development. These services are applied across therapeutic areas such as oncology, metabolic disorders, neurological disorders, hematology, infectious diseases, and others, serving both adult and pediatric patient populations.
The orphan drugs service market research report is one of a series of new reports that provides orphan drugs service market statistics, including orphan drugs service industry global market size, regional shares, competitors with an orphan drugs service market share, detailed orphan drugs service market segments, market trends and opportunities, and any further data you may need to thrive in the orphan drugs service industry. This orphan drugs service market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The orphan drugs service market includes revenues earned by entities through clinical trial management, pharmacovigilance and safety monitoring, distribution and supply chain services, patient support and access programs, and data management and biostatistics services. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values and are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Orphan Drugs Service Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on orphan drugs service market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for orphan drugs service? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The orphan drugs service market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Report Scope
Markets Covered:
1) By Type: Congenital Disease; Genetic Disease; Tumors And Cancer; Chronic; Other Types2) By Therapeutic Area: Oncology; Metabolic Disorders; Neurological Disorders; Hematology; Infectious Diseases; Other Rare Diseases
3) By Application: Aldult; Children
Subsegments:
1) By Congenital Disease: Heart Defects; Cleft Lip And Palate; Spina Bifida; Down Syndrome2) By Genetic Disease: Cystic Fibrosis; Huntington Disease; Sickle Cell Anemia; Muscular Dystrophy
3) By Tumors And Cancer: Leukemia; Lymphoma; Neuroblastoma; Sarcoma
4) By Chronic: Rare Metabolic Disorders; Rare Endocrine Disorders; Rare Cardiovascular Disorders; Rare Neurological Disorders
5) By Other Types: Rare Infectious Diseases; Rare Immunological Disorders; Rare Dermatological Conditions; Rare Ophthalmological Disorders
Companies Mentioned: Johnson & Johnson Services Inc.; F. Hoffmann-La Roche Ltd.; Pfizer Inc.; AbbVie Inc.; Bayer AG; Sanofi S.A.; Novartis AG; AstraZeneca PLC; The Bristol-Myers Squibb Company; GSK plc.; Eli Lilly and Company; Takeda Pharmaceutical Company Limited; Amgen Inc.; IQVIA Inc.; CSL Behring GmbH; Lonza Group AG; BioMarin Pharmaceutical Inc.; Fortrea Holdings Inc.; Medpace Inc.; Sarepta Therapeutics; Caidya Inc.; Cevidra Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Orphan Drugs Service market report include:- Johnson & Johnson Services Inc.
- F. Hoffmann-La Roche Ltd.
- Pfizer Inc.
- AbbVie Inc.
- Bayer AG
- Sanofi S.A.
- Novartis AG
- AstraZeneca PLC
- The Bristol-Myers Squibb Company
- GSK plc.
- Eli Lilly and Company
- Takeda Pharmaceutical Company Limited
- Amgen Inc.
- IQVIA Inc.
- CSL Behring GmbH
- Lonza Group AG
- BioMarin Pharmaceutical Inc.
- Fortrea Holdings Inc.
- Medpace Inc.
- Sarepta Therapeutics
- Caidya Inc.
- Cevidra Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | October 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 43.4 Billion |
| Forecasted Market Value ( USD | $ 80.62 Billion |
| Compound Annual Growth Rate | 17.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 23 |