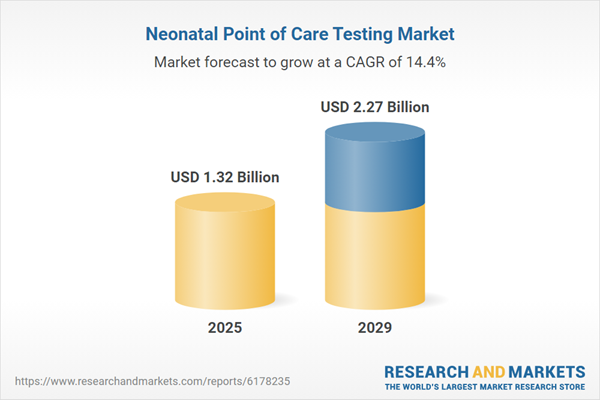
The neonatal point of care testing market size is expected to see rapid growth in the next few years. It will grow to $2.27 billion in 2029 at a compound annual growth rate (CAGR) of 14.4%. Growth in the forecast period is expected to be supported by rising demand for personalized neonatal care, increased adoption of portable testing devices, growing preference for early disease detection, heightened focus on reducing neonatal mortality rates, and greater investment in advanced diagnostic tools. Key trends anticipated for the forecast period include advancements in biosensor technology, innovation in microfluidic-based diagnostic platforms, increased research and development for multiplex assays, development of wireless neonatal monitoring systems, and innovation in point-of-care molecular testing.
The growing demand for remote patient monitoring is anticipated to support the expansion of the neonatal point of care testing market. Remote patient monitoring refers to the use of digital technology to provide healthcare services by connecting patients with medical professionals without requiring in-person visits. This demand is rising due to the need for timely, accessible care, particularly in remote and underserved areas where medical infrastructure may be limited. Neonatal point of care testing plays a key role in remote monitoring by delivering immediate diagnostic results at the bedside, allowing healthcare providers to rapidly identify and address critical health conditions in newborns without depending on central laboratories. For example, in February 2023, a survey conducted by Rock Health, a health technology company based in the United States, showed that seventy-six percent of respondents over the age of fifty-five had used telemedicine, while eighty percent of all participants had accessed healthcare through telemedicine at some point in their lives, up from seventy-two percent in 2021. Therefore, the rising demand for remote patient monitoring is driving growth in the neonatal point of care testing market.
Leading companies in the neonatal point of care testing market are forming strategic collaborations to expand access to diagnostics and improve early healthcare for newborns. Strategic collaborations involve partnerships between organizations that combine their resources and expertise to reach shared objectives. For example, in January 2025, QIAGEN, a provider of sample and assay technologies based in the Netherlands, entered a partnership with Genomics England, a genomic medicine organization located in the United Kingdom. This collaboration supported the launch of the Generation Study, a project aimed at sequencing the genomes of one hundred thousand newborns in England to identify more than two hundred treatable genetic conditions. QIAGEN is contributing clinically relevant genetic variant data for the genes assessed in the point of care sequencing test, enabling faster analysis and reporting of results. The study officially began in October 2024 and is expected to impact the early detection and treatment of conditions affecting approximately three thousand newborns each year in the United Kingdom.
In March 2025, LaCAR MDx Technologies, a biotechnology company based in Belgium, acquired the newborn screening division of Baebies Incorporated for an undisclosed amount. This acquisition expands LaCAR's portfolio by increasing the range of neonatal diagnostic solutions it can offer and supports the company’s growth in the United States market. Baebies Incorporated is a company based in the United States that specializes in neonatal point of care testing.
Major players in the neonatal point of care testing market are Abbott Laboratories, Siemens Healthineers AG, Koninklijke Philips N.V., GE Healthcare Technologies Inc., Konica Minolta Inc., Dräger Ag Co. Kg, Bio-Rad Laboratories Inc., Masimo Corporation, QIAGEN Inc., Radiometer Ltd., Contec Medical Systems Co. Ltd., Owen Mumford Ltd., Nova Biomedical, Heal Force Bio Meditech Holdings Ltd., Edan Instruments Inc., Trivitron Healthcare, Bistos Co. Ltd., Bilimetrix S.r.I, GINEVRI srl, Hadleigh Health Technologies.
North America was the largest region in the neonatal point of care testing market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in neonatal point of care testing report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the neonatal point of care testing market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The fast surge in U.S. tariffs and the trade tensions that followed in spring 2025 are heavily affecting the medical equipment sector, particularly for imported imaging machine components, surgical-grade stainless steel, and plastic disposables. Hospitals and clinics resist price hikes, pressuring manufacturers’ margins. Regulatory hurdles compound the problem, as tariff-related supplier changes often require re-certification of devices, delaying time-to-market. Companies are mitigating risks by dual-sourcing critical parts, expanding domestic production of commoditized items, and accelerating R&D in cost-efficient materials.
Neonatal point-of-care testing is the practice of performing diagnostic evaluations near the location where newborns receive care. It provides immediate clinical information, enabling rapid and informed decision-making. This approach delivers timely and accurate results while reducing the delays and logistical challenges associated with traditional laboratory testing.
The primary products in neonatal point-of-care testing include instruments and assays. Instruments consist of portable analyzers, handheld readers, and bedside monitors used to assess newborn health directly in care settings. Test types include neonatal bilirubin testing, newborn metabolic screening, blood glucose testing, infectious disease screening, electrolyte testing, and others. Key end-users include hospitals, diagnostic centers, maternity and specialty clinics, and additional healthcare facilities.
The neonatal point of care testing market research report is one of a series of new reports that provides neonatal point of care testing market statistics, including neonatal point of care testing industry global market size, regional shares, competitors with a neonatal point of care testing market share, detailed neonatal point of care testing market segments, market trends and opportunities, and any further data you may need to thrive in the neonatal point of care testing industry. This neonatal point of care testing market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The neonatal point of care testing market consists of revenues earned by entities by providing services such as rapid diagnostic screening, blood gas and electrolyte analysis, glucose level monitoring, and sepsis detection. The market value includes the value of related goods sold by the service provider or included within the service offering. The neonatal point of care testing market also includes sales of portable diagnostic analyzers, oxygen saturation monitors, hematology analyzers, and lactate analyzers. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Neonatal Point Of Care Testing Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on neonatal point of care testing market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for neonatal point of care testing? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The neonatal point of care testing market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Report Scope
Markets Covered:
1) By Product: Instruments; Assay2) By Test Type: Neonatal Bilirubin Testing; Newborn Metabolic Screening; Blood Glucose Testing; Infectious Disease Screening; Electrolyte Testing; Other Test Types
3) By End-Users: Hospitals; Diagnostic Centers; Maternity And Specialty Clinics; Other End-Users
Subsegments:
1) By Instruments: Blood Gas Analyzers; Bilirubinometers; Glucose Meters; Hematology Analyzers; Multiparameter Monitors2) By Assay: Bilirubin Assays; Glucose Assays; Infectious Disease Assays; Hematology Assays; Cardiac Marker Assays; Electrolyte Assays; Blood Gas Assays
Companies Mentioned: Abbott Laboratories; Siemens Healthineers AG; Koninklijke Philips N.V.; GE Healthcare Technologies Inc.; Konica Minolta Inc.; Dräger Ag Co. Kg; Bio-Rad Laboratories Inc.; Masimo Corporation; QIAGEN Inc.; Radiometer Ltd.; Contec Medical Systems Co. Ltd.; Owen Mumford Ltd.; Nova Biomedical; Heal Force Bio Meditech Holdings Ltd.; Edan Instruments Inc.; Trivitron Healthcare; Bistos Co. Ltd.; Bilimetrix S.r.I; GINEVRI srl; Hadleigh Health Technologies.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Neonatal Point of Care Testing market report include:- Abbott Laboratories
- Siemens Healthineers AG
- Koninklijke Philips N.V.
- GE Healthcare Technologies Inc.
- Konica Minolta Inc.
- Dräger Ag Co. Kg
- Bio-Rad Laboratories Inc.
- Masimo Corporation
- QIAGEN Inc.
- Radiometer Ltd.
- Contec Medical Systems Co. Ltd.
- Owen Mumford Ltd.
- Nova Biomedical
- Heal Force Bio Meditech Holdings Ltd.
- Edan Instruments Inc.
- Trivitron Healthcare
- Bistos Co. Ltd.
- Bilimetrix S.r.I
- GINEVRI srl
- Hadleigh Health Technologies.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | October 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.32 Billion |
| Forecasted Market Value ( USD | $ 2.27 Billion |
| Compound Annual Growth Rate | 14.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |