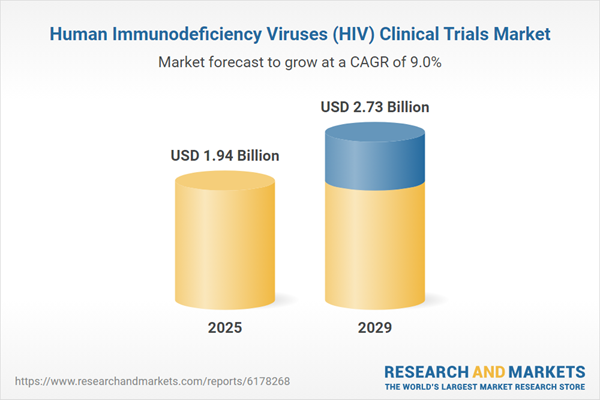
The human immunodeficiency viruses (HIV) clinical trials market size is expected to see strong growth in the next few years. It will grow to $2.73 billion in 2029 at a compound annual growth rate (CAGR) of 9%. The growth in the forecast period is driven by the increasing prevalence of HIV infections, rising awareness of HIV prevention and treatment, growing adoption of antiretroviral therapy, increased government funding and support for HIV research, and higher investment in biotechnology and pharmaceutical sectors. Key trends in the forecast period include technology-driven innovations in HIV diagnostics, advancements in antiretroviral therapy (ART) formulations, developments in biomarker identification, technology-enabled remote monitoring tools, and integration of digital health platforms.
The increasing number of human immunodeficiency virus infections is expected to drive the growth of the human immunodeficiency virus clinical trials market. Human immunodeficiency virus infections occur when the virus attacks and weakens the immune system, making individuals more vulnerable to infections and potentially progressing to acquired immunodeficiency syndrome. The rise in HIV cases is primarily attributed to insufficient awareness regarding transmission methods, which leads to high-risk behaviors and delays in testing, thereby facilitating the spread of the virus. Human immunodeficiency virus clinical trials play a vital role in addressing this issue by evaluating new drugs, vaccines, and treatment approaches to enhance prevention, improve disease management, and reduce transmission rates and disease progression. For example, in August 2024, the Minority HIV/AIDS Fund, a government agency based in the United States, reported that in 2023, an estimated 39.9 million people globally were living with HIV, including 38.6 million adults and 1.4 million children, reflecting an increase compared to previous years. Therefore, the growing incidence of HIV infections is contributing to the expansion of the human immunodeficiency virus clinical trials market.
Companies operating in the human immunodeficiency virus clinical trials market are focusing on developing long-acting injectable treatments to improve prevention strategies and reduce the rate of new infections. An HIV prevention injection is a long-acting medication administered at regular intervals to high-risk individuals to prevent infection by inhibiting the virus from establishing itself in the body. For example, in August 2025, Gilead Sciences Inc., a pharmaceutical company based in the United States, received approval from the European Union for Yeytuo, also known as lenacapavir, for the treatment of HIV. Yeytuo is a twice-yearly injectable prevention treatment that has shown nearly one hundred percent efficacy in clinical trials, significantly reducing the likelihood of acquiring HIV-1 through sexual transmission. The extended dosing schedule improves patient adherence and convenience when compared to daily oral pre-exposure prophylaxis, helping to address a significant gap in current prevention methods. This treatment is intended for adults and adolescents at increased risk, with the goal of expanding access and supporting global efforts to lower new HIV infection rates as part of a comprehensive prevention framework.
In July 2025, Elliott Investment Management L.P., an investment company based in the United States, along with Patient Square Capital LP and Veritas Capital Fund Management L.L.C., both health-focused investment firms based in the United States, completed the acquisition of Syneos Health for seven point one billion United States dollars. Through this acquisition, the investment firms aim to accelerate the growth of Syneos Health, improve customer service, and transform the organization into a technology-driven leader in biopharmaceutical solutions. Syneos Health is a pharmaceutical company based in the United States that conducts human immunodeficiency virus clinical trials.
Major players in the human immunodeficiency viruses (HIV) clinical trials market are Johnson & Johnson, F. Hoffmann-La Roche Ltd., Pfizer Inc., AbbVie Inc., Sanofi S.A., Thermo Fisher Scientific Inc., Amgen Inc., Gilead Sciences Inc., Merck & Co., IQVIA Holdings Inc., ICON plc, Moderna Inc., ViiV Healthcare Limited, HOOKIPA Pharma Inc., Excision BioTherapeutics Inc., American Gene Technologies International Inc., Bionor Holding AS, Aelix Therapeutics, Immuno Cure BioTech Private Limited, ImmunityBio.
North America was the largest region in the human immunodeficiency viruses (HIV) clinical trials market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in human immunodeficiency virus (HIV) clinical trials report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the human immunodeficiency virus (HIV) clinical trials market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the consequent trade frictions in spring 2025 are severely impacting the healthcare sector, particularly in the supply of critical medical devices, diagnostic equipment, and pharmaceuticals. Hospitals and healthcare providers are facing higher costs for imported surgical instruments, imaging equipment, and consumables such as syringes and catheters, many of which have limited domestic alternatives. These increased costs are straining healthcare budgets, leading some providers to delay equipment upgrades or pass on expenses to patients. Additionally, tariffs on raw materials and components are disrupting the production of essential drugs and devices, causing supply chain bottlenecks. In response, the industry is diversifying sourcing strategies, boosting local manufacturing where possible, and advocating for tariff exemptions on life-saving medical products.
Human immunodeficiency virus (HIV) clinical trials are research studies conducted on humans to evaluate the safety, effectiveness, and potential outcomes of new drugs, therapies, vaccines, or treatment strategies for preventing, managing, or curing HIV infection. These trials follow structured phases, ranging from early safety testing to large-scale efficacy studies, and provide the clinical evidence required for regulatory approval and enhanced patient care.
The primary phases of human immunodeficiency virus (HIV) clinical trials are Phase I, Phase II, Phase III, Phase IV, and preclinical. Phase I represents the first stage of testing, conducted with a small group of healthy volunteers or patients. These trials examine drug types such as antiretroviral therapy (ART), pre-exposure prophylaxis (PrEP), post-exposure prophylaxis (PEP), long-acting injectable antiretrovirals, and experimental drugs. Study designs include interventional studies, observational studies, and expanded access studies. Targeted patient populations include adults, pediatric patients, seniors, pregnant women, and high-risk groups. End-users of the results include pharmaceutical companies, research institutions, hospitals, and related organizations.
The human immunodeficiency virus (HIV) clinical trials market research report is one of a series of new reports that provides human immunodeficiency virus (HIV) clinical trials market statistics, including human immunodeficiency virus (HIV) clinical trials industry global market size, regional shares, competitors with a human immunodeficiency virus (HIV) clinical trials market share, detailed human immunodeficiency virus (HIV) clinical trials market segments, market trends and opportunities, and any further data you may need to thrive in the human immunodeficiency virus (HIV) clinical trials industry. This human immunodeficiency virus (HIV) clinical trials market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The human immunodeficiency virus (HIV) clinical trials market includes revenues earned by entities by providing services such as clinical study design and protocol development, patient recruitment and screening, and medical monitoring and care. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Human Immunodeficiency Viruses (HIV) Clinical Trials Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on human immunodeficiency viruses (hiv) clinical trials market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for human immunodeficiency viruses (hiv) clinical trials? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The human immunodeficiency viruses (hiv) clinical trials market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Report Scope
Markets Covered:
1) By Phase: Phase I; Phase II; Phase III; Phase IV; Preclinical2) By Drug Type: Antiretroviral Therapy (ART); Pre-Exposure Prophylaxis (PrEP); Post-Exposure Prophylaxis (PEP); Long-Acting Injectable Antiretrovirals; Experimental Drugs
3) By Study Design: Interventional Studies; Observational Studies; Expanded Access Studies
4) By Patient Population: Adults; Pediatric Patients; Seniors; Pregnant Women; High-Risk Populations
5) By End User: Pharmaceutical Companies; Research Institutes; Hospitals; Other End-Users
Subsegments:
1) By Phase I: First-In-Human Studies; Safety And Tolerability Studies2) By Phase Ii: Dose-Finding Studies; Efficacy Studies
3) By Phase Iii: Pivotal Trials; Comparative Efficacy Studies
4) By Phase Iv: Post-Marketing Surveillance; Long-Term Safety Studies
5) By Preclinical: In Vitro Studies; Animal Studies
Companies Mentioned: Johnson & Johnson; F. Hoffmann-La Roche Ltd.; Pfizer Inc.; AbbVie Inc.; Sanofi S.A.; Thermo Fisher Scientific Inc.; Amgen Inc.; Gilead Sciences Inc.; Merck & Co.; IQVIA Holdings Inc.; ICON plc; Moderna Inc.; ViiV Healthcare Limited; HOOKIPA Pharma Inc.; Excision BioTherapeutics Inc.; American Gene Technologies International Inc.; Bionor Holding AS; Aelix Therapeutics; Immuno Cure BioTech Private Limited; ImmunityBio
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Human Immunodeficiency Viruses (HIV) Clinical Trials market report include:- Johnson & Johnson
- F. Hoffmann-La Roche Ltd.
- Pfizer Inc.
- AbbVie Inc.
- Sanofi S.A.
- Thermo Fisher Scientific Inc.
- Amgen Inc.
- Gilead Sciences Inc.
- Merck & Co.
- IQVIA Holdings Inc.
- ICON plc
- Moderna Inc.
- ViiV Healthcare Limited
- HOOKIPA Pharma Inc.
- Excision BioTherapeutics Inc.
- American Gene Technologies International Inc.
- Bionor Holding AS
- Aelix Therapeutics
- Immuno Cure BioTech Private Limited
- ImmunityBio
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | October 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.94 Billion |
| Forecasted Market Value ( USD | $ 2.73 Billion |
| Compound Annual Growth Rate | 9.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |